Periodontitis is an inflammatory disease involving supportive structures of the teeth leading to progressive destruction of periodontal tissues, loss of attachment, aesthetics and ultimately loss of tooth, primary etiological factor being the bacterial plaque [1]. Different types of microorganisms are involved in different types of periodontal diseases [2]. P. gingivalis is considered as an important periodontal pathogen which is a member of red complex along with T.denticola and T.forsythia causing different types of periodontitis [3]. The main goal of periodontal treatment is to establish adequate infection control through disruption of the microbial biofilm and suppression of the inflammation and maintain the same [4]. Various antimicrobial agents have been used in the management of periodontal infection. Chlorhexidine (CHX) is most commonly employed antimicrobial agent for periodontal therapy [5]. Thorough mechanical debridement of plaque and repeated administration of systemic and topical antimicrobial agents has limited effectiveness against microorganisms involved in periodontal lesions [6]. Complex anatomy of tooth system, lack of accessibility to microorganisms in the periodontal pocket and increase in the number of resistant strains to the available antimicrobial agents often result in persistent and refractory infections [7,8]. The nature of periodontal destruction depends on virulence of microorganisms, duration of the disease and host defence mechanism [9]. Therefore, it is extremely important to design and develop newer approaches to overcome these limitations.
Nanotechnology is the application of science and technology to control matter at the molecular level. The properties of matter are significantly different from their macroscopic bulk properties at the nano-scale level [10]. Various Nanoparticles (NPs) have gained popularity owing to their unique mode of action, potent antimicrobial activity and provide long term effective treatment at the site of infection at much smaller doses [11]. AgNPs are considered to be the best antimicrobial agents owing to their broad spectrum of activity and biocompatibility. Therefore, it offers an attractive alternative to conventional antimicrobial agents [12].
Currently, there is a growing need for using environment friendly nanoparticles which do not produce toxic wastes in their synthesis, not being hazardous to human health and environment. To achieve this, nanotechnology has inclined to shift to biosynthetic process. The use of biological entities like bacteria, fungi, herbal extracts and yeasts in the synthesis of AgNPs is a relatively novel method [13]. Microorganisms play an important role in remediation of toxic metals through reduction of the metal ions and minimize the toxicity during the process of NPs production by formation of insoluble complexes with metal ions [14]. Fungi has many advantages over other microorganisms as they require simple nutrients to grow, the process of biosynthesis using fungi is simple, easy, economical, covers large surface areas by suitable growth of the fungi and allows for development of reliable processes for NPs synthesis over a range of sizes with good monodispersity and chemical composition hence, considered as naturally occurring nano-factories [11,15]. Studies have been reported on application of synthetic NPs for periodontal therapy [6,16]. However, the literature is scarce regarding the application of biosynthesised AgNPs in periodontal therapy [17]. This article aims at producing AgNPs using the endophytic fungi fusarium semitectum, characterization by visual observation, UV-Vis spectrophotometer, TEM, SAED, FTIR and evaluating the antibacterial efficacy using the agar diffusion method against the periodontal pathogen P. gingivalis.
Materials and Methods
The present in-vitro study was conducted in the Department of Periodontics, HKE’s SN Institute of Dental Sciences and Research, Kalaburgi, India. Biosynthesis of AgNPs and assessment of antibacterial activity conducted at Department of Microbiology, Gulbarga University, Kalaburgi, India.
Endophytic fungi were isolated from healthy fresh leaves of Withania Somnifera (Ashwagandha) collected from Department of Botany, Gulbarga University, Kalaburgi. The leaves were gently washed several times under running tap water and cut into small pieces. The cut surface was sterilized by immersing into 70% ethanol for 30 seconds followed by 0.01% mercuric chloride for 5 minutes and sodium hypochlorite for another three minutes and finally rinsed with distilled water and blot dried using filter paper. Cut surface of the leaf was placed on a petridish containing Potato Dextrose Agar (PDA) supplemented with streptomycin sulfate (250 mg/ml) and incubated at 280C for six days. The fungi were isolated and pure cultures were prepared by inoculating on freshly prepared PDA plates and identified based on morphological and microscopic characteristics.
For biosynthesis of AgNPs, fresh cultures of fungi were inoculated in Erlenmeyer flask of 100 ml MGYP broth and incubated at 29°C for 72 hours for the biomass to grow. Fungal biomass was harvested, filtered using Whatman filter paper number 1 and washed several times with sterile distilled water. A 25 gm of biomass (wet weight) was placed in flasks containing 100 ml of sterile distilled water and incubated for 48 hours. The filtrate mixed with aqueous solution of AgNO3 of 1mM concentration kept in dark at 29°C for 24 hours were used for further experiments.
Characterization of biosynthesized AgNPs was initially monitored by visual observation for colour change of the solution. The test solution with AgNO3 and the control solution without AgNO3 were monitored for the colour change for 24 hours. The formation of AgNPs was further monitored by absorbance peaks recorded at wavelengths between 200 nm to 600 nm using a double beam UV–Vis Spectrophotometer (Victoria, Australia).
Morphology, particle size, shape and crystal structure of AgNPs were determined using high resolution TEM (JEOL/JEM 2100, USA). The samples were prepared by transferring aliquot of aqueous suspension of AgNPs onto a carbon coated copper grid and air dried under vacuum. TEM micrographs were taken at 100 kV. The crystalline nature of AgNPs was analysed and confirmed by TEM using SAED pattern.
The presence of biomolecules was determined using FTIR spectrophotometer (Thermo Nicolet Avatar 370, USA). The biosynthesized AgNPs were centrifuged at 10000 rpm for 15 minutes, the pellets were resuspended in distilled water and microcentrifuged at 10000 rpm for another 15 minutes. The pellets were air dried at room temperature and the collected powder form was subjected for scanning using FTIR spectrophotometer. Briefly, 2 mg of powder sample was mixed with 200 mg Potassium bromide (KBr) (FTIR grade), pressed into a pellet and placed in the sample holder. FTIR spectrum was scanned in the range 4000–400 cm-1 at a resolution of 4 cm-1 and the images were analysed.
The antibacterial efficacy of AgNPs was evaluated against P. gingivalis by agar well diffusion method as per National Committee for Clinical Laboratory Standards (NCCLS) guidelines [18,19]. The P. gingivalis (ATCC-33277) strains (American Type Culture Collection, Manassas, VA, USA) were subcultured from the stock cultures grown and maintained on sterilized trypticase soya agar base supplemented with 5% sheep blood, hemin (5 μg/ml), vitamin K1 (0.5 μg /ml) and yeast extract (10 mg/ml). The agar plates were incubated at 37°C for 72 hours under anaerobic atmosphere (10% CO2, 5% H2, and 85% N2). The colonies were confirmed by gram staining and biochemical tests.
Inoculum was prepared by transferring the colonies from the plates with a sterilized straight nichrome wire to the trypticase soya broth supplemented with 5% sheep blood, hemin (5 g/ml), vitamin K1 (5 g/ml) and yeast extract (1 mg/ml) and incubated at 37°C in anaerobic conditions overnight. The density of the microbial suspension equal to 0.5 Mc Farland constant was adjusted to 1.5×108 CFU/mL by using a spectrophotometer. Entire surface of the supplemented TSA plate was swabbed three times, to ensure even distribution the plates were rotated approximately 60° between streaking. The wells of 5 mm diameter were prepared using a sterile punching device in the agar plate. A (20 μl), B (40 μl), C (60 μl), D (80 μl) and E (100 μl) of AgNPs, F (0.2% CHX), G (2% CHX), H (Ampicillin) and I (sterile distilled water) were added into respective wells in the agar plates. The plates were incubated for 18-24 hours at 37°C anaerobically. The incubated plates were read only after the confluent growth. Diameter of the zone of inhibition (clear area surrounding each well) was measured using the calipers to the nearest millimeters. The results of inhibition zones were interpreted according to CLSI documentation, diameter of zone > 17 mm is considered to be susceptible and </= 13mm is considered to be resistant [20]. The experiment was carried out six times for each solution and the results were tabulated accordingly.
Statistical Analysis
All the data required for this study were collected and analysed statistically using One-way ANOVA and Tukey post-hoc multiple comparison tests to analyse the zone of inhibition of antibacterial efficacy.
Results
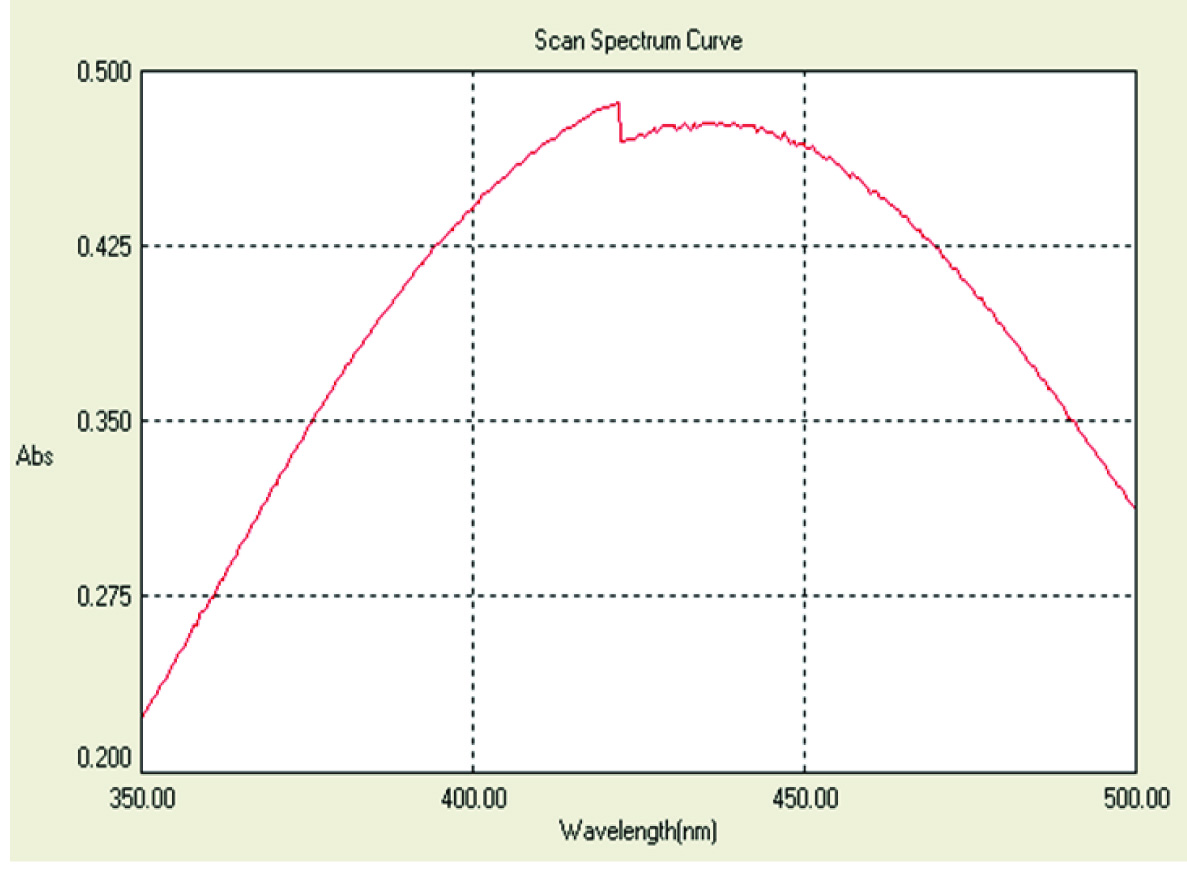
Based on the microscopic and macroscopic characteristics, the fungal isolate was identified as Fusarium semitectum [Table/Fig-1]. Visual observation of colour change from colourless to reddish brown colour was noticed after completion of the reaction. The control (without AgNO3) showed no colour change of the cell free filtrate when incubated under the same conditions [Table/Fig-2]. UV-Vis spectrum showed the maximum absorbance peak at 420 nm after 24 hours of incubation [Table/Fig-3].
Fusarium semitectum on PDA plates.

Visual observation of colour change from colour less to reddish brown.

UV-V is spectrum showing peak at 420 nm.
TEM micrographs revealed that AgNPs are spherical in shape, uniformly distributed without significant agglomeration and most of the particles are 10 nm-20 nm in size [Table/Fig-4]. The nanocrystalline nature of AgNPs was confirmed by TEM using SAED. The SAED image showed diffraction lattice pattern in the silver region in the form of rings [Table/Fig-5].


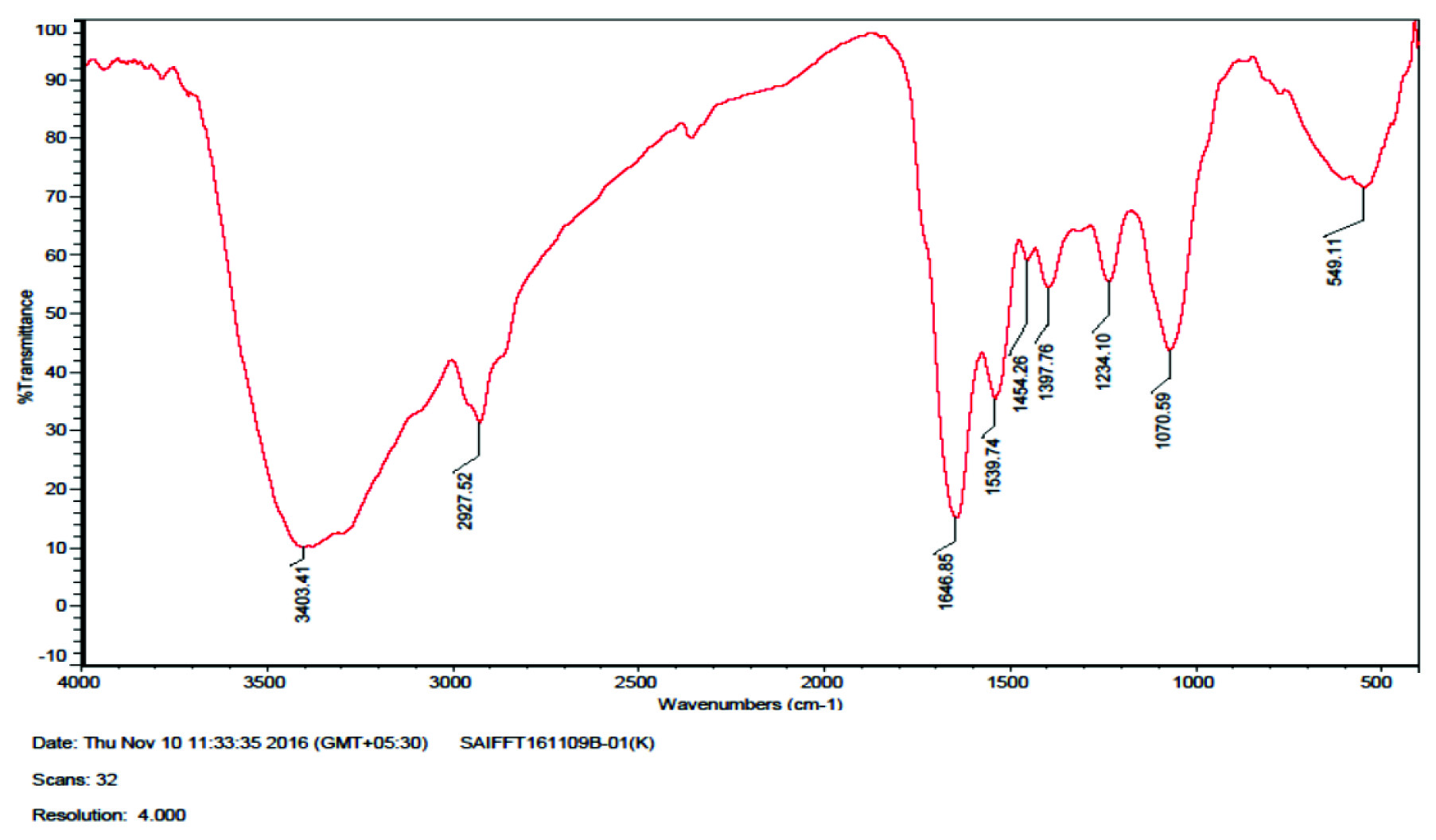
FTIR spectrum shows the presence of eight bands at 1646.85, 1539.74, 2927.52, 1397.76, 1234.10, 1454.26, 1077.59, 3403 cm-1 [Table/Fig-6]. The 1646.85 and 1539.74 cm-1 correspond to the binding vibrations of amide I and amide II due to carbonyl stretch and N–H stretch vibrations in the amide linkages of the proteins, while 2927.52 cm-1 represents CH2 stretch vibrations of alkane linkages. The bands observed at 1397.76, 1454.26, 1234.10 and 1077.59 cm-1 represent the CH2 and C-N stretching vibrations of aromatic and aliphatic amines and the corresponding N-H stretching vibrations of the primary amines were observed at 3403 cm-1. These observations indicate the binding of fungal derived proteins with AgNPs.
AgNPs D (80 μl) and E (100 μl) showed mean zone of inhibition of 17.33 mm and 18 mm, F (CHX 0.2%) and G (CHX 2%) showed 17.85 mm and 19.97 mm. Positive control H (Ampicillin) 20.5 mm and no zone of inhibition for the negative control group I (sterile distilled water) [Table/Fig-7]. Tukey post-hoc multiple comparisons show no significant difference between D and F, E and F, E and G. (p<0.001) [Table/Fig-8]. Therefore, biosynthesized AgNPs (80 μl and 100 μl) are as effective as 0.2 CHX and 2 % CHX against P.gingivalis.
Mean, standard deviation of zone of inhibition and One-way ANOVA.
| Variables | N | MEAN | Standard Deviation (SD) | 95% Confidence Interval for men | ANOVA F | p-value |
|---|
| Upperlimit | Lowerlimit |
|---|
| AgNPsA (20 ml) B (40 ml)C (60 ml)D (80 ml)E (100 ml) CHLORHEXIDINEF (0.2%)G (2 %)AMPICILLINHDISTILLED WATERI | 666666666 | 11.1013.9816.2817.3318.0017.8519.9720.5No Zone | 0.22800.04080.19410.258200.24820.78660 | 10.9313.9516.1417.14-17.6719.39- | 11.2714.0116.4317.52-18.0320.54- | 551.32 | P<0.0001 |
Multiple comparision by Tukey post-hoc test of antibacterial efficacy.
| Between | p-value |
|---|
| A - BCDEFGH | <0.0010<0.0010<0.0010<0.0010<0.0010<0.0010<0.0010 |
| B - CDEFGH | <0.0010<0.0010<0.0010<0.0010<0.0010<0.0010 |
| C - DEFGH | <0.0010<0.0010<0.0010<0.0010<0.0010 |
| D - EFGH | 0.01920.1318<0.0010<0.0010 |
| E - FGH | 0.89990.0752<0.0010 |
| F - GH | <0.0010<0.0010 |
| G - H | 0.1399 |
Discussion
The main causative factor of the periodontal diseases are the microorganisms inhibiting the periodontal pockets [2,21]. Periodontal pockets provide suitable environment for survival and growth of the microorganisms residing in the pockets [22,23]. The abundance and diversity of microorganisms depends upon several factors such as: effectiveness of oral hygiene measures, pocket depth, flow of gingival crevice fluid, degree of gingivitis, type of interacting microbes and viruses, host immune response, emergence of new microorganisms and development of resistance to most of the available antibiotics [24]. Therefore, it is essential to develop new antimicrobial agents with higher efficacy while being non invasive, non toxic and without drug resistance and achieve high success rate [25]. Application of AgNPs as antimicrobial agents alternative to existing antimicrobial agents in periodontal therapy is a method that can overcome these limitations [26].
AgNPs can be synthesized by different chemical and physical approaches [27]. Concern has been raised on the toxicity of chemicals used, cost and technical issues in synthesis of AgNPs. Biomimetic approaches provide a novel idea for production of NPs without using hazardous substances to human health and environment [13]. Recent studies have shown the synthesis of AgNPs using fungi and antibacterial efficacy against several microbes [13,28]. Characterization of AgNPs is essential for better understating of morphology, size and dimensions of the materials in nano range [26]. Visual observation is the primary indication of the formation of AgNPs. The change in colour after addition of AgNO3 to the fungal filtrate indicates the reduction of the AgNO3 by the fungal derived nitrate reductase enzyme which occurs due to excitation of surface plasmon resonance [11]. Biotransformation of AgNPs was further monitored using UV-Vis spectrum. AgNPs possess specific optical properties and therefore, interact with certain wavelengths of light. The conduction and valence bands in AgNPs lie very close to each other in which electrons move freely and give rise to Surface Plasmon Resonance (SPR) absorption band due to collective oscillation of electrons of AgNPs in resonance with the light waves hence, UV spectra shows the band at 420 nm. The possible mechanism suggests the reduction of silver ions is mainly due to a conjugation between the electron -shuttle with the fungal derived nitrate reductase enzyme [15].
TEM provides the quantitative measures of particle size, size distribution, morphology and gives better spatial resolution [29]. Size and shape of the AgNPs depends on the type of the microorganisms and factors such as the temperature and pH of the medium therefore, considerable size variability can be seen of AgNPs produced by different fungal species. Size ranges of 5–60 nm, 5–25 nm and 5–35 nm have been reported for Fusarium sp [28,30].
FTIR is used to identify the role of biological molecules involved in the reduction of silver nitrate to silver ions and to analyse the binding of proteins with AgNPs and characterize the secondary structures involved in the metal NPs-fungal derived protein interactions [29]. It is confirmed from the different FTIR bands that amino acid and peptides form a coat covering the AgNPs preventing agglomeration; secondary structures of the proteins were not affected as a consequence of the reaction with silver ions and act as stabilization and capping agents.
Most investigations of biosynthesized AgNPs for antimicrobial activity have used the agar diffusion method [28,30]. The results of the present study showed the antibacterial activity of AgNPs against p gingivalis with maximum zone of inhibition of 18 mm and CHX 0.2% and CHX 2% showed 17.85 mm and 19.97 mm respectively. Therefore, biosynthesized AgNPs are as effective as CHX against P.gingivalis . Bahadoor A et al., reported mean zone of inhibition of 9 mm and 13 mm against P.gingivalis of nanosilver Iranian MTA (NS-IMTA) produced using fungi Aspergillus terreus [17].
The mechanism of the antibacterial activity of AgNPs involves the reaction between the positively charged AgNPs and negatively charged membrane of the microorganisms [31]. The mechanism includes the release of Ag ions, disruption of bacterial cell membrane, DNA damage and other factors such as the concentration, size and shape of particles also affect the efficacy against the microorganisms [32]. AgNPs possess high antimicrobial properties owing to their increased surface area, biocompatibility and the shape of crystals causes numerous highly reactive corners [33]. AgNPs show higher affinity for Gram-negative and anaerobic bacteria which is related to lower peptidoglycan content in Gram-negative bacteria. The difference in affinity for aerobic and anaerobic bacteria is attributed to the ability of AgNPs binding to the enzymes containing sulfhydryl (SH) groups [34].
Chlorhexidine (CHX) as a gold standard chemical agent is the most effective antimicrobial agent used since 1950’s as an oral antiseptic in mouthwash, toothpaste, and chewing gum. Its effectiveness can be attributed to it antibacterial effects and its substantivity within the oral cavity. For plaque control, it is recommended to use 0.2% to 0.12% CHX [5]. A 2% CHX is recommended as a potential irrigant owing to its unique ability to bind to dentin, and its substantivity in the root canal system and relative absence of cytotoxicity [35]. Hence, both 0.2% and 2% CHX are used in the present study.
Today, we come across the treatment failures even after using the contemporary treatment modalities therefore, new treatment strategies have to be developed to control the disease process and to achieve high success rate [26]. The application of biosynthesized AgNPs in present study shows an insight for research in periodontal therapy as an alternative to topical antiseptics and antimicrobial agents can be used in combination with other antimicrobial agents as synergetic effect and for local drug delivery during periodontal therapy [26].
Limitation
Despite of the common application of agar diffusion method, many factors apart from the actual antibacterial activity of the material tested might affect the accuracy and reproducibility of the agar diffusion method. These factors include the solubility and diffusion of the material through the agar medium, contact between the gel and test material, thickness of the gel, agar viscosity, storage conditions of the agar plates and incubation time, application procedures might also affect the diffusion of the material into the agar. However, this study is a basic approach, other methods of evaluating the antimicrobial activity and further in vivo and in vitro studies should be considered in future for the effective use of these particles.
Conclusion
The ability to synthesize AgNPs as potential antimicrobial agents derived from endophytic fungi is highly promising for the sustainable green synthesis of AgNPs and its antibacterial efficacy against P gingivalis enhances its widespread application as antimicrobial agent as an important strategy in the treatment of periodontal diseases.