Introduction
Maintenance of original canal anatomy with proper disinfection is our primary goal to achieve during root canal instrumentation. Surfactants are added to irrigating solution to promote deeper penetration into dentinal tubules.
Aim
The aim of this study was to evaluate the influence of addition of surfactants to Sodium Hypochlorite (NaOCl) and Ethylenediaminetetraacetic Acid (EDTA) on transportation of root canal.
Materials and Methods
Fifty human mandibular molars with mesial root curvatures of 10° – 40° were selected and embedded in silicone impression material to simulate mandibular arch form to facilitate imaging process and maintain reproducibility of images. Before instrumentation, root canals were scanned by using Cone Beam Computed Tomography (CBCT) imaging (Carestream, India). The canals were then prepared with the ProTaper Next (PTN) system (Dentsply Maillefer, Ballaigues, Switzerland), using one of the following irrigation regimens during the instrumentation and were divided into five groups based on irrigation regimens followed: G1 (n=10)-irrigation with saline solution(control); G2 (n=10)-irrigation with 2.5% NaOCl; G3 (n=10)-irrigation with 2.5% NaOCl added with surfactant; G4 (n=10)-irrigation with 17% EDTA; G5 (n=10)-irrigation with 17% EDTA added with surfactant. Post-instrumentation scans were obtained with similar parameters and position as pre-instrumentation scans by CBCT imaging. Transportation of the root canals were then analysed at three cross-sectional planes of pre-instrumentation and post-instrumentation images at 2 mm, 5 mm, 8 mm from the apical end of the root. The data was statistically analyzed using Analysis of Variance (ANOVA) and Tukey post hoc test (p<0.05).
Results
The mean transportation values were higher in G5. Transportation in G3 and G5 was not significantly different compared to G2 and G4 respectively (p<0.05).
Conclusion
Instrumentation using irrigating solutions added with surfactant like 1% cetrimide maintained the canal curvature well.
Introduction
The root canal system has multiple geometric planes and curve significantly more than the roots that house them [1]. Maintenance of original canal anatomy with proper debridement during root canal instrumentation of curved canals remains a challenge, as there will be more dentin removal in one direction than symmetrical removal which leads to root canal transportation [2]. There will be accumulation of debris, microorganisms, necrotic and decayed tissue in the areas of root canal where there is inadequate instrumentation [3]. So instrumentation must always be supported by the use of antimicrobial irrigating solutions for cleaning the areas of root canal system which are not directly affected by instrumentation [4].
Of different irrigation solutions used during instrumentation, NaOCl has both antimicrobial and proteolytic activity which dissolves necrotic tissue [5]. and organic components of the smear layer [6]. While EDTA is a chelating agent that removes calcium ions to demineralise the inorganic component of smear layer created by root canal instrumentation [7]. But despite of their excellent properties, these irrigants are not capable of easily reaching canal irregularities. So to achieve deeper penetration into dentinal tubules and canal anatomic complexities, surface modifiers are added to irrigation solutions to reduce surface tension [8-11].
Surface modifiers also called as surfactants or surface active agents act as detergents, emulsifiers, wetting agents, foaming agents or dispersants [12-13]. reduce the surface tension of irrigants and enhance their microbial effectiveness and improve clinical performance when added to them [14-16].
However, several studies indicated that irrigants can modify original properties of dentine like permeability, solubility and decrease microhardness [17] by altering the chemical structure of dentine, thus changing the calcium/phosphorous ratio of dentine [18,19]. NaOCl reduces microhardness of dentin regardless of concentrations [20,21] and EDTA solution induces a decrease in root dentin microhardness [9,22,23]. Surfactant like cetrimide itself caused decrease in microhardness of dentin which was due to its tetrahedral structure [24]. So change in microhardness of dentine could even compromise the maintenance of original root canal path which could lead to canal transportation.
There are numerous studies on evaluation of maintenance of path in curved canals using different instrumentation and preparation techniques [25-27]. There are only two studies which evaluated irrigation solutions on canal transportation [28,29]. But none of the studies evaluated the association of surface modifiers to irrigation solutions on canal transportation. Thus, the present study aimed to evaluate addition of cetrimide as surface modifier to NaOCl and EDTA on canal transportation using CBCT. The null hypothesis was that addition of surfactants to irrigants increases canal transportation.
Materials and Methods
This in vitro study was conducted at G. Pulla Reddy Dental College and Hospital, Kurnool, Andhra Pradesh, India. Human mandibular first and second molars with normal root morphology extracted for the reasons of periodontal and prosthodontic considerations were selected. Inclusion criteria for selection were teeth with completely formed apices and two distinct mesial root canals. Exclusion criteria were teeth with root caries, cracks, resorption, calcification and incomplete apices. The tissue and debris were removed from root surfaces with hand curettes and stored in saline at 4°C until use. Each tooth was radiographed in the mesiodistal and buccolingual planes to ensure two mesial canals with independent foramen. Teeth with root caries, cracks, resorption and incomplete apices, ≤10 mm root length were excluded. Fifty teeth with Mesiobuccal (MB) root curvature of 10°-40° determined using Schneiders technique [30] were selected as samples.
Specimen preparation
Endodontic access cavities were prepared using a round diamond bur (Dentsply Maillefer) and MB canal orifice was explored with a ISO size 10 K-file which was passively advanced into the canal until it was visible at the apical foramen. Working Length (WL) was established by subtracting 1 mm from this length. All the teeth crowns were flattened to standardize the length to 16 mm. Teeth were fixed in silicone impression material, which was simulated in mandibular arch form to facilitate imaging process and maintain reproducibility of images. Pre-instrumented root canals were scanned by using CBCT (Carestream, India) imaging with 10×5 Field of View (FOV). Scout views by CBCT machine automatically determines the tube potential and tube current and were kept constant before and after instrumentation images. 3D image acquisition was performed using the CBCT CS 9300 (90 kV, 6.3 mA) at high resolution dental mode. CBCT images of the sample were analyzed with CS 9300 imaging software for the 3D multiplanar reconstruction and measurements.
Randomization and Experimental Groups
After initial scans specimens were then categorised by simple random sampling into five experimental groups (n=10) according to the irrigation regimen used.
Group 1 (G1) n=10-irrigation with 5 ml of 0.9% saline between files.
Group 2 (G2) n=10-During instrumentation, the canal was filled with 3 ml of 2.5 % NaOCl solution. In between each instrument change, the canal was rinsed with 2 ml of 0.9% saline.
Group 3 (G3) n=10-During instrumentation, the canal was filled with 3 ml of 2.5 % NaOCl added with 1% cetrimide solution. In between each instrument change, the canal was rinsed with 2 ml of 0.9% saline.
Group 4 (G4) n=10-During instrumentation, the canal was filled with 3ml of 17 % EDTA solution. In between each instrument change, the canal was rinsed with 2 ml of 0.9% saline.
Group 5 (G5) n=10-During instrumentation, the canal was filled with 3 ml of 17% EDTA added with 1% cetrimide solution. In between each instrument change, the canal was rinsed with 2 ml of 0.9% saline.
All the specimens were instrumented using Nickel Titanium (NiTi)PTN system at a rotational speed of 300 rpm and 2.5 N/cm torque according to the manufacturer’s instructions with an electrical motor (X-Smart, Dentsply Maillefer) and a 16:1 reduction handpiece. The final instrumentation for each group was F3. All the instruments were used up to the WL. Each instrument was used in three canals. All the liquid irrigants were delivered using a 30-G, side-vented needle (Max-i-Probe; Dentsply Rinn, Elgin, IL).
After instrumentation post-instrumentation scans were obtained with similar parameters and position as pre-instrumentation scans by CBCT imaging. Canal transportation of the root canals was then analysed at three cross-sectional planes of pre-instrumentation and post-instrumentation images at 2 mm, 5 mm, 8 mm from the apical end of the root. Transportation at each level was calculated using the following formula (a1-a2)-(b1-b2), where a1 is the shortest distance between mesial aspect of non instrumented canal to mesial edge of the root, and a 2 is the shortest distance between mesial aspect of instrumented canal to the mesial edge of the root. Likewise b1 is the shortest distance between distal aspect of non instrumented canal to distal edge of theroot, and b 2 is the shortest distance between distal aspect of instrumented canal to distal edge of the root [31]. The result 0 indicated no canal transportation, negative results means distal transportation and positive results show mesial transportation.
Statistical Analysis
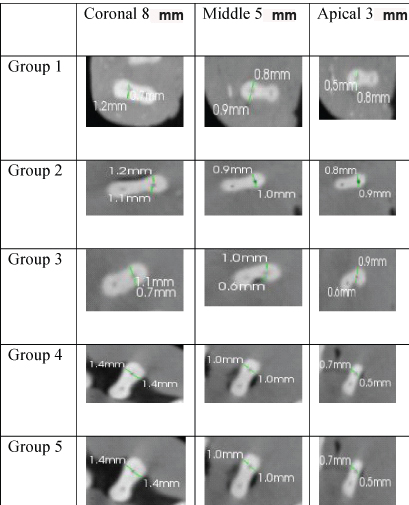
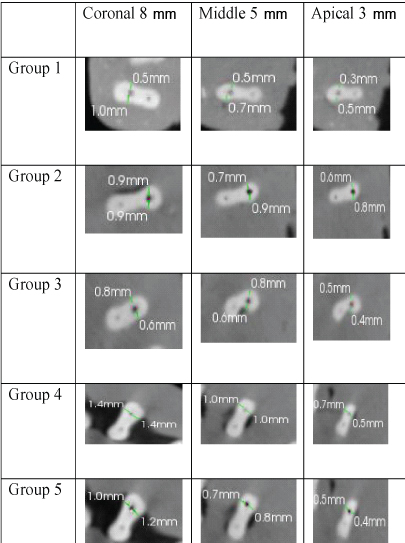
Data were statistically ANOVA and Tukey post hoc test for comparison among groups. The significance level was set at p=0.05. Statistical analysis was performed with SPSS Statistics Version 18.0 for Windows. [Table/Fig-1,2] shows the pre-instrumentation and post-instrumentation CBCT images of five groups at three different levels.
Representative pre-instrumentation CBCT images of the root cross-section at 8 mm, 5 mm, 3 mm from apex.
Representative post-instrumentation CBCT images of the root cross-section at 8 mm, 5 mm, 3 mm from apex.
Results
[Table/Fig-3] shows the mean values of transportation of five groups at three different levels. On comparison of these five groups at 3 mm, 5 mm and 8 mm from apex with respect to transportation scores by one way ANOVA, the results had showed that there was no significant difference between the five groups at any level (p>0.05). On comparison of three levels within each group there was significant difference with p<0.05. On pairwise comparison by Tukey’s post hoc test coronal values are significantly higher than apical values (p>0.05).
Mean values of transportation of five groups at three different levels.
| Groups | 8 mm | 5 mm | 3 mm | p-value | Post-hoc test |
|---|
| Mean | SD | Mean | SD | Mean | SD |
|---|
| Group1 | 0.16 | 0.07 | 0.08 | 0.08 | 0.06 | 0.07 | 0.012; Sig | 8>3 |
| Group2 | 0.17 | 0.09 | 0.08 | 0.08 | 0.07 | 0.08 | 0.025; Sig | 8>3 |
| Group3 | 0.15 | 0.11 | 0.10 | 0.08 | 0.07 | 0.09 | 0.18; NS | - |
| Group4 | 0.18 | 0.08 | 0.09 | 0.11 | 0.08 | 0.07 | 0.033; Sig | 8>3 |
| Group5 | 0.25 | 0.07 | 0.10 | 0.10 | 0.07 | 0.07 | <0.001; Sig | 8>5,3 |
| p-value | 0.088; NS | 0.974; NS | 0.987; NS | | |
A p-value of <0.05 was considered statistically significant.
ANOVA with post-hoc Tukey’s test.
Discussion
Cameron JA was the first to report that surface modifiers when added to NaOCl enhanced their ability to dissolve organic material [8]. Surfactants bind primarily to the Ca2+ and Mg2+ ions in water, which decreases detergency and foam [13,32]. Cetrimide {cetyltriethyl ammonium bromide (CTAB)} is both a disinfecting agent and a cationic surfactant, reduces the surface tension of the irrigant [10,33], improves antibacterial effectiveness [14,15], facilitates penetration of the irrigant to the dentin surface [9], and increases the wettability of the dentin surface [10].
In addition, cetrimide may also alter the structure of hydroxyapatite nanorods in a concentration dependent manner. It also elongates the hydroxyapatite nanorods, thereby the length diameter ratio of these nanorods decreases when the content of CTAB increases potentially altering the physical properties of dentin [34]. It was previously reported that a 0.5% cetrimide solution decreased dentin microhardness to a similar extent as 5% EDTA [24]. Change in microhardness of dentine could even compromise the maintenance of original root canal path which could lead to canal transportation. Transportation may occur because of the physical properties of the files or as a result of chelating agents or irrigants used during canal preparation.
Present study results showed most apical transportation measurements were 0.1 mm and had not exceeded 0.3 mm. According to Peters canal transportation of up to 0.10 mm is acceptable [35]. Conversely, canal transportation above 0.30 mm may have a negative impact on the root filling, affecting the results of treatment [36] and none of the group apical values exceeded this limit in the present study. In the G1 of the present study, saline was used during instrumentation and transportation has occurred in few specimens in this group, though saline has no chelating ability and only removes debris created during instrumentation. This might be due to mechanical properties of the file as all instrumentation technique will invariably lead to removal of some dentin from root canal walls [1]. In G2 and G3, irrigation was conducted with NaOCl and NaOCl added with cetrimide respectively. Transportation values of G2, G3 are higher compared to G1 but no significant difference between three groups. This might be because NaOCl decreases microhardness of dentin [20,21,37] as it dissolved not only collagen component of dentine but also magnesium and phosphate ions while increasing the amount of dentinal carbonate [38,39] which significantly altered the ca/p ratio [19]. These changes in dentin mineralization may affect the hardness profile [40] and lead to transportation. In G4 and G5, irrigation was conducted with EDTA and EDTA added with cetrimide respectively. Canals prepared with 17% EDTA solution showed greater transportation values with no difference between groups. High transportation values might be because EDTA is a strong chealator, and by its chelating action, it binds to calcified components (particularly +2 ions) of dentin causing demineralization and softening of dentin. These results are consistent with a molecular analysis of chelating agents applied to root dentin in which neutral EDTA extracted significantly more calcium and phosphorus from the coronal two thirds of the root [41]. The increased demineralization occurring in the specimens prepared with 17% EDTA solution probably facilitated the greater transportation observed in these group mainly in the coronal third. These results are consistent with a previous study where experimental groups prepared with 17% EDTA solution showed greater increase transportation than those prepared with RC-Prep [29].
There was also no significant difference in canal transportation when EDTA was added with 1% cetrimide. It might be because cetrimide does not affect the extraction properties of EDTA. The results of our study was supported by a previous study where ca2+ extraction properties of EDTA did not differ significantly compared with EDTA + cetrimide treated for 10 minutes and 15 minutes [42]. G5 showed highest values compared to other groups, it is due to addition of 1% cetrimide which had enhanced the efficacy of irrigation solution by decreasing the surface tension and increasing the wettability causing easy spread over dentin surface, thereby improving irrigation efficiency. Our results showed that 1% cetrimide addition to irrigating solutions does not significantly increase the canal transportation.
Limitation
Limitation of the study was irrigation activation devices were not used which might influence the fluid penetration into dentinal tubules. Futures studies have to be conducted to evaluate the use of irrigation activation devices and surfactants on canal transportation
Conclusion
On basis of methodology used in the study and results obtained, it can be concluded that irrigating solutions added with 1% cetrimide as surfactant used during instrumentation of curved canals had not effected the transportation of original canal significantly compared to instrumentation using irrigating solutions without surfactant. Thus, instrumentation using irrigating solutions added with surfactant like 1% cetrimide maintained the canal curvature well.
A p-value of <0.05 was considered statistically significant.
ANOVA with post-hoc Tukey’s test.
[1]. Schilder H, Cleaning and shaping the root canalDent Clin North Am 1974 18:269-96. [Google Scholar]
[2]. Hartmann MS, Barletta FB, Camargo Fontanella VR, Vanni JR, Canal transportation after root canal instrumentation: a comparative study with computed tomographyJ Endod 2007 33:962-65. [Google Scholar]
[3]. Weine FS, Kelly RF, Lio PS, The effect of preparation procedures on original shape and apical foramen shapeJ Endod 1975 1:255-62. [Google Scholar]
[4]. Qing Y, Akita Y, Kawano S, Kawazu S, Yoshida T, Sekine I, Cleaning efficacy and dentin micro-hardness after root canal irrigation with a strong acid electrolytic waterJ Endod 2006 32:1102-06. [Google Scholar]
[5]. Naenni N, Thoma K, Zehnder M, Soft tissue dissolution capacity of currently used and potential endodontic irrigantsJ Endod 2004 30:785-87. [Google Scholar]
[6]. Haikel Y, Gorce F, Allemann C, Voegel JC, In vitro efficiency of endodontic irrigation solutions on protein desorptionInt Endod J 1994 27:16-20. [Google Scholar]
[7]. Hulsmann M, Heckendorff M, Lennon A, Chelating agents in root canal treatment: mode of action and indications for their useInt Endod J 2003 36:810-30. [Google Scholar]
[8]. Cameron JA, The effect of a fluorocarbon surfactant on the surface tension of the endodontic irrigant, sodium hypochlorite: a preliminary reportAust Dent J 1986 31:364-68. [Google Scholar]
[9]. Abou-Rass M, Patonai FJ Jr, The effects of decreasing surface tension on the flow of irrigating solutions in narrow root canalsOral Surg Oral Med Oral Pathol 1982 53:524-26. [Google Scholar]
[10]. Yılmaz Z, Aktemur S, Buzoglu HD, Gumusderelioglu M, The effect of temperature and pH variations on the surface tension of EDTA solutionsJ Endod 2011 37:825-27. [Google Scholar]
[11]. Palazzi F, Morra M, Mohammadi Z, Grandini S, Giardino L, Comparison of the surface tension of 5.25% sodium hypochlorite solution with three new sodium hypochlorite-based endodontic irrigantsInt Endod J 2012 45:129-35. [Google Scholar]
[12]. Schreier S, Malheiros SV, de Paula E, Surface active drugs: self association and interaction with membranes and surfactants—physicochemical and biological aspectsBiochim Biophys Acta 2000 1508:210-34. [Google Scholar]
[13]. Holmberg K, Jonsson B, Kronberg B, Lindman B, Surfactants and polymers in aqueous solution 2002 2nd edWest Sussex, EnglandJohn Wiley & Sons:01-66. [Google Scholar]
[14]. Rossi-Fedele G, Prichard JW, Steier L, de Figueiredo JA, The effect of surface tension reduction on the clinical performance of sodium hypochlorite in endodonticsInt Endod J 2013 46:492-98. [Google Scholar]
[15]. Ferrer-Luque CM, Conde-Ortiz A, Arias-Moliz MT, Valderrama MJ, Baca P, Residual activity of chelating agents and their combinations with cetrimide on root canals infected with Enterococcus faecalisJ Endod 2012 38:826-28. [Google Scholar]
[16]. Wang Z, Shen Y, Ma J, Haapasalo M, The effect of detergents on the antibacterial activity of disinfecting solutions in dentinJ Endod 2012 38:948-53. [Google Scholar]
[17]. Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, Zalkind M, Histochemical analysis of dental hard tissues following bleachingJ Endod 1996 22:23-25. [Google Scholar]
[18]. Hennequin M, Pajot J, Avignant D, Effects of different pH values of citric acid solutions on the calcium and phosphorus contents of human root dentinJ Endod 1994 20:551-54. [Google Scholar]
[19]. Dogan H, Qalt S, Effects of chelating agents and sodium hypochlorite on mineral content of root dentinJ Endod 2001 27:578-80. [Google Scholar]
[20]. Ari H, Erdemir A, Belli S, Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentinJ Endod 2004 30:792-95. [Google Scholar]
[21]. Slutzky-Goldberg I, Maree M, Liberman R, Heling I, Effect of sodium hypochlorite on dentin microhardnessJ Endod 2004 30:880-82. [Google Scholar]
[22]. Rotstein I, Effect of hydrogen peroxide and sodium perborate on the microhardness of human enamel and dentineJ Endod 1994 20:61-63. [Google Scholar]
[23]. Saquy PC, Maia Campos G, Sousa Neto MD, Guimaraes LF, Pecora JD, Evaluation of chelating action of EDTA in association with Dakin’s solutionBraz Dent J 1994 5:65-70. [Google Scholar]
[24]. Akcay I, Sen BH, The effect of surfactant addition to EDTA on microhardness of root dentinJ. Endod 2012 38(5):704-07. [Google Scholar]
[25]. Aguiar CM, De Andrade Mendes D, Camara AC, De Figueiredo JA, Evaluation of the centreing ability of the ProTaper universal rotary system in curved roots in comparison to Nitiflex filesAust Endod J 2009 35:174-79. [Google Scholar]
[26]. Kunert GG, Camargo Fontanella VR, De Moura AA, Barletta FB, Analysis of apical root transportation associated with ProTaper universal F3 and F4 instruments by using digital subtraction radiographyJ Endod 2010 36:1052-55. [Google Scholar]
[27]. Oliveira CA, Meurer MI, Pascoalato C, Silva SR, Conebeam computed tomography analysis of the apical third of curved roots after mechanical preparation with different automated systemsBraz Dent J 2009 20:376-81. [Google Scholar]
[28]. Silva e Souza PA, das Dores RSE, Tartari T, Pinheiro TPS, Tuji FM, Silva e Souza MH Jr, Effects of sodium hypochlorite associated with EDTA and etidronate on apical root transportationInt Endod J 2014 47(1):20-25. [Google Scholar]
[29]. Whitbeck ER, Swenson K, Tordik PA, Kondor SA, Webb TD, Sun J, Effect of EDTA preparations on rotary root canal instrumentationJ Endod 2015 Jan 41(1):92-96. [Google Scholar]
[30]. Schneider SW, A comparison of canal preparations in straight and curved root canalsOral Surg Oral Med Oral Pathol 1971 32:271-75. [Google Scholar]
[31]. Gambill JM, Alder M, del Rio CE, Comparison of nickel-titanium and stainless steel hand-file instrumentation using computed tomographyJ Endod 1996 22:369-75. [Google Scholar]
[32]. Huibers PDT, Lobanov VS, Katritzky AR, Shah DO, Karelson M, Prediction of critical micelle concentration using a quantitative structure property relationship approachJ Colloid Interface Sci 1997 187:113-20. [Google Scholar]
[33]. Zehnder M, Schicht O, Sener B, Schmidlin P, Reducing surface tension in endodontic chelator solutions has no effect on their ability to remove calcium from instrumented root canalsJ Endod 2005 31:590-92. [Google Scholar]
[34]. Tianyuan M, Zhigou X, Libing L, Effect of reaction systems and surfactants additives on the morphology evaluation of hydroxyapatite nanorods obtained via a hydrothermal routeAppl Surf Sci 2011 257:4384-88. [Google Scholar]
[35]. Peters OA, Current challenges and concepts in the preparation of root canal systems: a reviewJ Endod 2004 30:559-67. [Google Scholar]
[36]. Wu MK, Fan B, Wesselink PR, Leakage along apical root fillings in curved root canals. Part I: effects of apical transportation on seal of root fillingsJ Endod 2000 26(4):210-16. [Google Scholar]
[37]. Oliveira LD, Carvalho CA, Nunes W, Valera MC, Camargo CH, Jorgo AO, Effects of clorhexidine and sodium hypochlorite on the microhardness of root canal dentinOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 104:e125-28. [Google Scholar]
[38]. Sayin TC, Serper A, Cehreli ZC, Otlu HG, The effect of EDTA, EGTA, EDTAC and tetracycline-HCl with and without subsequent NaOCl treatment on the microhardness of root canal dentinOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 104:418-24. [Google Scholar]
[39]. Tsuda H, Ruben J, Arends J, Raman spectra of human dentin mineralEur J Oral Sci 1996 104:123-31. [Google Scholar]
[40]. Arends J, ten Bosch JJ, Demineralization and remineralization evaluation techniquesJ Dent Res 1992 71:924-28. [Google Scholar]
[41]. Verdelis K, Eliades G, Oviir T, Margelos J, Effect of chelating agents on the molecular composition and extent of decalcification at cervical, middle and apical root dentin locationsEndod Dent Traumatol 1999 15:164-70. [Google Scholar]
[42]. Gupta R, Gupta P, Gupta S, Gupta T, Effect of cetrimide in decalcifying efficacy of irrigating solutionsInternational Journal of Applied Dental Sciences 2015 1(3):35-37. [Google Scholar]