Benign Lesions on Screening Mammography: Increasing Diagnostic Confidence in a Hitherto Unscreened Population
Piyush Joshi1, Rohit Sharma2
1 Associate Professor, Department of Radiology, INHS Asvini, Mumbai, Maharashtra, India.
2 Associate Professor, Department of Radiology, INHS Asvini, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Piyush Joshi, Associate Professor, Department of Radiology, INHS Asvini, Colaba, Mumbai-400005, Maharashtra, India.
E-mail: piyushjoshi@hotmail.com
Introduction
Screening mammography is used for detection of breast cancer and is interpreted using the Breast Image Reporting and Data System (BIRADS) convention. It is not routinely offered to women in countries which do not have a national screening programme resulting in a challenge for the radiologist who has to interpret these in the absence of previous mammograms.
Aim
To analyse benign and probably benign lesions in screening mammograms in a hitherto unscreened population and suggest protocols to increase diagnostic confidence.
Materials and Methods
Screening mammograms of 362 asymptomatic women in the age range of 40 to 60 years carried out over a two year period were retrospectively analysed. Patients of breast cancer and those who had palpable lumps were excluded from the study. All images were analysed in standard Mediolateral Oblique (MLO) and Craniocaudal (CC) projections with additional views wherever necessary. Corroborative ultrasound had been carried out wherever indicated in the opinion of the interpreting radiologist. The mammograms were finally classified according to the BIRADS convention.
Results
Of the total number of 362 women screened, most of whom did not have any previous mammogram, 162 were reported as BIRADS I, 179 as BIRADS II and 18 as BIRADS III. The mammograms reported as BIRADS II had various findings including dystrophic calcification/macrocalcification, vascular calcification, simple cysts and fibroadenomas. Only 26 (16.04%) of the BIRADS I mammograms had undergone further evaluation with Ultrasound (US) due to dense breasts or asymmetrical involution of breast tissue whereas 76 (42.5%) of the BIRADS II mammograms had undergone further evaluation with US to characterize lesions like cysts and fibroadenomas, but occasionally also for benign clustered calcification. Of BIRADS III mammograms, 12 (66.6%) had required US correlation to exclude a mass in cases with dense breasts. The increased likelihood of ultrasound corroboration in BIRADS II and BIRADS III was analysed using the Chi square test and was statistically significant.
Conclusion
In the absence of previous screening mammograms, a small number of BIRADS I mammograms and a significant number of BIRADS II and BIRADS III mammograms undergo a corroborative US examination. The addition of supplemental US to the evaluation of these lesions increase diagnostic confidence and lesion characterization in a population which is not subject to routine screening.
Breast cancer, Mass Screening, Mediolateral oblique
Introduction
Mammographic screening for breast cancer is routinely offered to women in selected age groups in many western countries [1]. Mammograms are universally interpreted using the BIRADS convention which was developed by the American College of Radiology in 1993 [2]. During mammographic screening, a large number of benign (BIRADS II) or likely benign (BIRADS III) lesions are also incidentally detected. Often these are the only lesions detected and sometimes these can be seen in addition to another primary lesion (BIRADS IV or BIRADS V). Some workers [3] have reported a prevalence of probably benign lesions to be close to 50% out of which the vast majority (49%) was BIRADS II and a small number (0.57%) were BIRADS III. Thus, in a mammographic screening programme, the vast majority of lesions detected are likely to be benign or probably benign.
According to the World Health Organisation (WHO), breast cancer is the second most common cancer in females in India with a Crude Incidence Rate (CIR) of 39.7 per 105 populations in the age group of 35–64 years. Women have a lifetime risk of 1:40 of developing breast cancer [4].
A screening mammogram is one in which the woman does not have any symptoms of breast disease as opposed to a diagnostic mammogram where a patient presents with symptoms of breast disease and the examination is intended to evaluate any such abnormality [5].
Mammographic screening is to detect unsuspected or undetected disease which could be malignant and for this to be successful, disease has to be detected at an earlier stage in order to affect mortality. Analysis of current and older studies together enables the radiologist to pick up subtle variations in the architecture thereby enabling early detection.
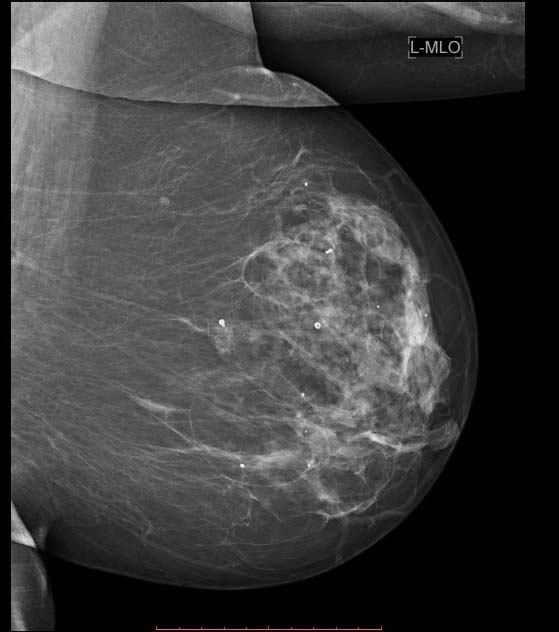
There are a large number of lesions that can be classified as definitely benign. These include lucent-centered calcifications, skin calcifications, diffusely scattered calcifications [Table/Fig-1], milk of calcium, vascular calcifications, fat necrosis [Table/Fig-2] etc. The appearance of dense large calcifications, especially within a typical lobulated mass, are supposed be diagnostic of a benign involuting fibroadenoma [Table/Fig-3]. In the initial period, these calcifications may be very small and irregular and may be difficult to distinguish from malignant microcalcification. At this time, biopsy may be indicated. Later the calcifications coalesce to give a typical appearance described as popcorn calcification [6].
Mammogram Left breast MLO view showing scattered macrocalcifications;
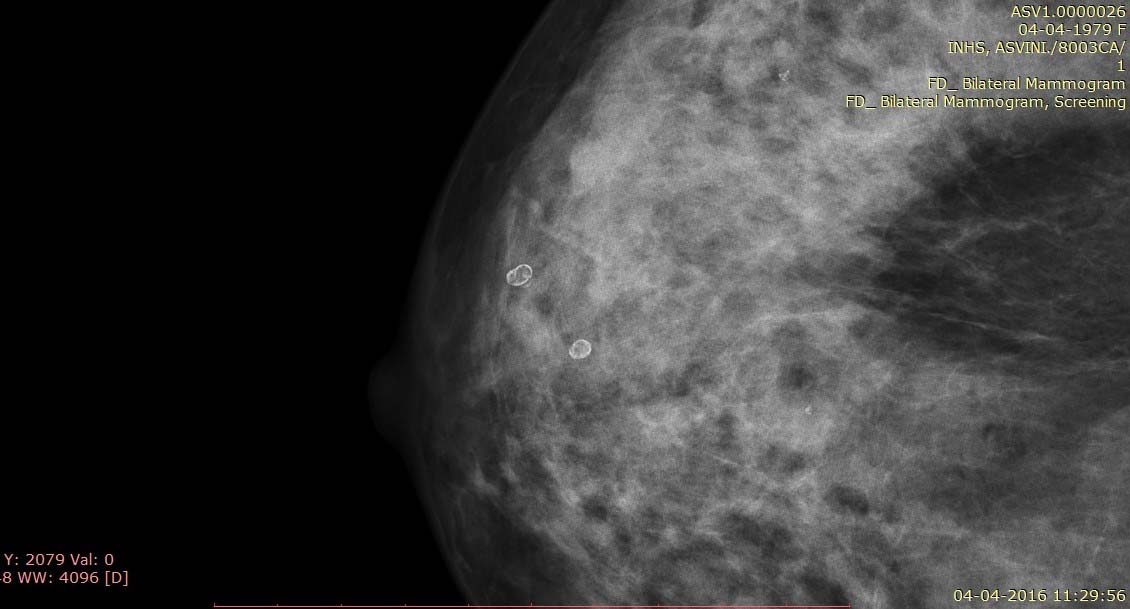
Mammogram of the right breast craniocaudal view shows lucent lesions with rim calcification representing oil cysts.
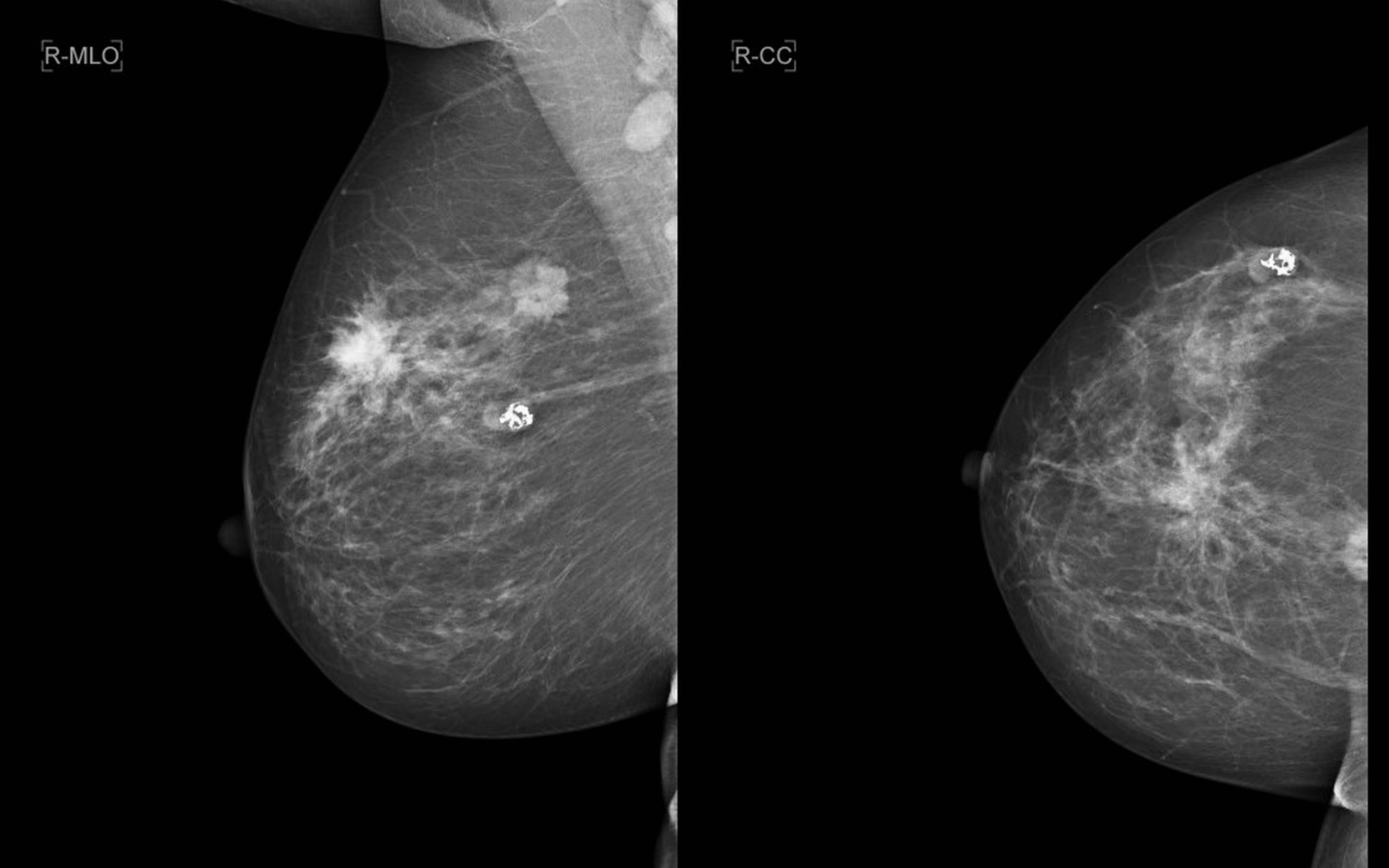
a). Right breast mediolateral oblique; b) craniocaudal views reveal two mass lesions one demonstrating spiculation and the other microcalcification likely malignant etiology. Also, seen is a well defined mass with “popcorn” calcification typical of an involuting fibroadenoma.
One of the greatest challenges in mammography is the detection and management of a lesion that is probably benign. These lesions are almost always benign but on less than 2% occasions can subsequently turn out to be malignant. These lesions should not be biopsied but are to be followed up based on cost benefit analysis. Subsequently if they show any change in morphology with time, management can be altered later if there is a change in morphology.
In countries where there is no national screening programme, screening mammography is offered only to high risk patients or to women who opt for the same. The challenge and the dilemma for radiologists is to interpret these mammograms in the absence of the previous years’ mammograms for comparison and the possibility of the patient being lost to follow up. Therefore, the present study was conducted in interpreting benign and probably benign lesions in such mammograms and suggests possible adjuncts to the imaging algorithm.
Materials and Methods
A retrospective analysis was carried out of 362 screening mammograms at the Department of Radiology over a two-year period from May 2014 to April 2016 after obtaining clearance from the Institutional Ethical Committee. Images had been acquired on a digital mammography machine (Mammomat Novation DR, Siemens Healthineers, Germany). All these mammograms were of women who had opted for screening and did not have any symptoms. Women of age less than 40 or greater than 60 years were excluded from the study. Patients who had been treated for breast cancer were also excluded from the study. Also excluded from the study were patients with palpable lumps. Two projections of both breasts had been obtained-MLO and CC views. Specialised or magnification views were obtained wherever required. A corroborative US had been obtained wherever required in the opinion of the interpreting radiologist. These included dense breasts, all cases of non calcified lesions of solid density and lesions corresponding to BIRADS III. The mammograms were classified according to the BIRADS convention of the American College of Radiology [7]. The spectrum of benign and likely benign screening mammograms were analysed and assessed along with the requirement for a corroborative US examination.
Statistical Analysis
The total number of BIRADS I, BIRADS II and BIRADS III patients as well as the number of cases of these who underwent further corroborative US were analysed in a 3 X 2 contingency table using the Chi-square test to test the null hypothesis and assess whether there was a statistically significant association between the number of patients undergoing corroborative US and their BIRADS category.
Results
A total of 362 mammograms carried out over a two-year period were retrospectively analysed. The age distribution of these was 40 years to 59 years. In addition to the report which had been generated by the reporting radiologist, the mammograms were also independently reviewed by a radiologist with more than ten years’ experience in screening mammography. There was no difference between the reports of the two radiologists.
Of these mammograms, 162 were reported as BIRADS I, 179 as BIRADS II and 18 as BIRADS III. Additionally two cases were reported as BIRADS IV and one as BIRADS V. These findings are summarized in [Table/Fig-4]. Of the patients classified as BIRADS III, two were subjected to biopsy as they had a first degree relative with a history of breast cancer. The biopsy results in both these patients were negative for breast cancer. All other patients reported as BIRADS III underwent short interval follow up with two further mammograms at six month intervals and were finally classified as BIRADS II. None of these subsequently progressed to breast cancer over the follow up period. The mammograms reported as BIRADS II had various findings including dystrophic calcification/macrocalcification, vascular calcification, simple cysts and fibroadenomas. These findings are summarized in [Table/Fig-5].
Prevalence of various BIRADS categories in screening mammography series (n=362).
| BIRADS category | Prevalence (%) |
|---|
| BIRADS I | 162 (44.8) |
| BIRADS II | 179 (49.4) |
| BIRADS III | 18 (4.9) |
| BIRADS IV | 2 (0.6) |
| BIRADS V | 1 (0.3) |
Findings in mammograms classified as BIRADS II. Some mammograms had more than one findings.
| BIRADS II findings | Prevalence |
|---|
| Vascular calcification | 95 |
| Benign/dystrophic macrocalcification | 84 |
| Fat necrosis | 18 |
| Calcified cysts | 27 |
| Simple cysts | 12 |
| Fibroadenoma | 6 |
Of these mammograms, only 26 (16.04%) of the BIRADS I mammograms had required further evaluation with US due to dense breasts or asymmetrical involution of breast tissue whereas 76 (42.5%) of the BIRADS II mammograms had undergone further evaluation with US to characterize lesions like cysts and fibroadenomas, but occasionally also for benign clustered calcification. Of BIRADS III mammograms, 12 (66.6%) had undergone corroborative US to rule out a mass lesion in relatively dense breasts. Thus, it is apparent that in the absence of previous screening mammograms, a small number of BIRADS I mammograms and a significant number of BIRADS II and BIRADS III mammograms benefit from a corroborative US examination. The number of BIRADS I, BIRADS II and BIRADS III patients and the proportion of these groups which underwent further corroborative US were analysed testing the null hypothesis that there was no significant difference between these groups. The Chi-square test revealed a highly significant association between the likelihood of US corroboration being required in cases of BIRADS II and BIRADS III mammograms (benign and likely benign cases) with a p-value<0.001 .
Discussion
Analysis of benign and probably benign lesions is fundamental to increasing diagnostic efficacy in screening mammography as the remaining lesions by default are considered malignant or probably malignant. Now-a-days, breast radiologists accept the definition of probably benign lesions which has been given by Sickles EA [8] in his landmark paper. His categories included round or oval circumscribed masses; smooth, round, clustered microcalcifications; and focal asymmetric densities which did not represent masses. He employed magnification mammography to evaluate the margins of these lesions and the morphology of the microcalcifications. As long as the circumscribed lesions retained their sharp margins over 75% of the border, the remainder was obscured by normal tissues, the calcifications were round and regular, and the asymmetries did not form centrally dense masses fading toward their edges then it was seen that these lesions had only a 0.5% probability of developing malignant change [6].
In view of these findings Sickles EA had argued that management by mammographic surveillance is justified because probably benign lesions indeed have a very low likelihood of malignancy; mammographic surveillance will identify those few lesions that change in the interval that actually are malignant; and these cancers will still be diagnosed early in their course, while they still have a favorable prognosis [8].
The American College of Radiology (ACR) proposed a BIRADS to standardize mammography reports [7]. Orel SG et al., demonstrated that the placement of mammographic lesions into these BIRADS categories predicts the possibility of malignancy [9]. They also said that a lesion in BIRADS II is almost certainly benign and a lesion in BIRADS III is highly predictive of benignity; so much so that short interval follow up should be considered as an alternative to biopsy which would decrease the number of unnecessary biopsies performed in benign lesions. Additionally, Lazarus E et al., have reported good interobserver agreement amongst breast radiologists in using BIRADS lexicon and its use in prediction of malignancy [2]. Therefore, it can be said that following the BIRADS lexicon results in adequate management.
However, extrapolation of this data to populations where mammographic screening is not routinely offered is fraught with possible negative consequences. Firstly, in countries where mammographic screening is offered, it is considered purely a screening modality and not a diagnostic modality. Therefore, a negative (BIRADS I), benign (BIRADS II) and probably benign (BIRADS III) mammogram will be followed by further mammograms. The interval before these mammograms may differ, however. This would be true for all, with a small number being lost to follow up. The danger in populations where screening mammography is not routine is that the person might not voluntarily present for follow up, or may not present at the desired interval.
Western observers have also reported increase in the yield of cancers after supplementing mammography with US for high risk patients, though at the cost of increased false positives and increased number of biopsies. Berg WA et al., investigated the role of supplemental US in women with increased risk of breast cancer [10]. Increased breast cancer risk was defined after using a variety of criteria. A statistically significant increase in detection of breast cancer was identified with mammography and US combined as compared to mammography alone. The workers noted that such an increased yield may be due to a higher prevalence in the high risk group.
The role of supplemental US in women with dense breasts has also received a lot of interest in the past few years. A recent study by Okello J et al., studied 148 women prospectively over six months and noted that supplemental imaging by breast US detected 27% more malignant mass lesions [11]. The authors recommended routine US in such cases.
A much larger study was carried out by Chae EY et al., on 28796 women with dense breasts after dividing them into two distinct groups which were evaluated either by mammography alone or mammography and breast US [12]. They noted a markedly increased cancer detection yield in the second group. They recommended the addition of supplemental US to detect breast cancer in women who had dense breasts and were node negative. However, they added that this would also lead to an increased false positive rate.
This was also confirmed by a systematic review by Nothacker M et al., who analysed six cohort studies and concluded that addition of supplemental US identified increased number of small occult cancers although with an increased biopsy rate [13].
Unfortunately a literature search did not identify any study which focused on benign or probably benign lesions. Our study focused on this group and we found that addition of supplemental US increased diagnostic confidence among radiologists.
Limitation
The sample size is small. Further multicentric studies with a larger size would be required to plug this lacuna in the current knowledge on this aspect.
Conclusion
Screening mammography is not a routine investigation and as such radiologists are handicapped when interpreting these studies in a previously unscreened population as they do not have recourse to previous studies. The addition of corroborative US increases diagnostic confidence and improves lesion characterization and should be routinely resorted to in mammograms with lesions of atypical morphology or dense breasts.
[1]. Autier P, Ouakrim DA, Determinants of the number of mammographic units in countries with significant mammographic screeningBr J Cancer 2008 99(7):1185-90. [Google Scholar]
[2]. Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS, BI-RADS lexicon for US and mammography: interobserver variability and positive predictive valueRadiology 2006 239(2):385-91. [Google Scholar]
[3]. Milani V, Goldman SM, Finguerman F, Pinotti M, Ribeiro CS, Abdalla N, Presumed prevalence analysis on suspected and highly suspected breast cancer lesions in Sao Paulo using BIRADS criteriaSao Paulo Med J 2007 125(4):210-14. [Google Scholar]
[4]. Nair KM, Varghese C, Swaminathan R, Cancer: current scenario, intervention strategies and projections for 2015. In: National Commission on Macroeconomics and HealthBackground papers: Burden of disease in India 2005 New DelhiMinistry of Health and Family Welfare:219-225.Available from: http://www.who.int/macrohealth/action/NCMH_Burden%20of%20disease_(29%20Sep%202005).pdf [Google Scholar]
[5]. Poplack SP, Carney PA, Weiss JE, Titus-Ernstoff L, Goodrich ME, Tosteson AN, Screening mammography: Costs and use of screening related servicesRadiology 2005 234(1):79-85. [Google Scholar]
[6]. Kopans DB, Breast Imaging 2007 3rd edPhiladelphiaLippincott Williams & Wilkins [Google Scholar]
[7]. American College of Radiology [internet]. BI-RADS® Mammography 2013. Available from: http://www.acr.org/Quality-Safety/Resources/BIRADS/Mammography [Google Scholar]
[8]. Sickles EA, Periodic mammographic follow up of probably benign lesions: results in 3184 consecutive casesRadiology 1991 179(2):463-68. [Google Scholar]
[9]. Orel SG, Kay N, Reynolds C, Sullivan DC, BI-RADS categorization as a predictor of malignancyRadiology 1999 211(3):845-50. [Google Scholar]
[10]. Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancerJAMA 2008 299(18):2151-63. [Google Scholar]
[11]. Okello J, Kisembo H, Bugeza S, galukande M, Breast cancer detection using sonography in women with mammographically dense breastsBMC Medical Imaging 2014 14(14):41 [Google Scholar]
[12]. Chae EY, Kim HH, Cha JH, Shin HJ, Kim H, Evaluation of screening whole-breast sonography as a supplemental tool in conjunction with mammography in women with dense breastsJ Ultrasound Med 2013 32(9):1573-78. [Google Scholar]
[13]. Nothacker M, Duda V, Hahn M, Warm M, Degenhardt F, Madjar H, Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic reviewBMC Cancer 2009 9:335 [Google Scholar]