The incidence of snake-bites is high, but not all snake-bites are fatal. Chippaux JP reported that annually, total number of snake-bites might exceed five million, with snake-bite mortality of 1,25,000 in the world; four million snake-bites, two million snakebite envenomings, and 1,00,000 snake-bite deaths each year in Asia [1]. Kasturiratne A et al., reported that snake-bite with envenomation constitute 12% to 50% of the total bites in Asia and 18% to 30% in India and Pakistan [2]. In India, 66-163/1 lakh population suffer from snake bite annually with mortality of 1.1 to 2.4/1 lakh population and case fatality rate of 1.7% to 20% [1].
Recently available venom antigen based ELISA tests identify the species involved, but are expensive, not freely available and have limited diagnostic value [6]. Owing to absence of studies on venom levels and its modulation by various doses of ASV, the clinical dose of ASV dose for snake bite cases continues to be decided empirically [7]. The Indian and WHO guidelines clearly mention that ASV should be used judiciously, since it is relatively costly and in limited supply [4]. However, owing to fear of death, the treating physicians land up using the maximum mentioned dose of 10 vials ASV initially followed by 5-10 vials 6 hourly for every poisonous snake-bite case. This protocol is apparently liberal and often results in a critical shortage of ASV. During June 2013 to September 2013, when ASV was in short supply, our institute formulated a committee, having members from department of Medicine and Pharmacology, for judicious use of ASV. After a thorough study of the published literature, it was found that several studies [8-16] had tested various low dose regimens of ASV and reported that the low doses were not inferior to the higher dose. Syed MA et al., classified snake-bites based on manifestations and initial antivenom dose varied from 5-20 vials depending on severity [6]. The WHO guidelines of 2010 mention that initial dose of snake anti-venom varies from 1-10 vials based on the variety of snake [4]. Therefore, despite shortage of antivenom, following the published low dose protocols was not advisable. Our hospital statistics showed that cobra or saw-scaled viper were the most common variety of poisonous snake-bites. As per WHO guidelines, saw-scaled viper requires five vials as initial dose while neurotoxic manifestation of cobra-bite can be managed with neostigmine and ventilator support. Therefore, it was decided to formulate a modified protocol for clinical use at our institute, where initial dose of antivenom would be five vials. In order to incorporate the higher need for other species of snake-bite, there was a rescue protocol of higher dose (vide infra).
Having successfully implemented the modified ASV protocol during the period of short-supply, we performed a retrospective analysis to compare outcomes between modified and the conventional protocol in snake-bite cases.
The aim of the study was to obtain data on proportion of poisonous snake-bites among all snake-bite cases and to compare the modified low dose ASV protocol versus conventional ASV protocol with respect to outcome, average number of ASV vials required per patient, duration of hospitalization, Medical ICU (MICU) stay, additional interventions needed {including need for Fresh Frozen Plasma (FFP) and dialysis}.
Materials and Methods
This was a retrospective study conducted at a tertiary care teaching hospital, Pune, Maharashtra, India. The study protocol was approved by the Institutional Ethics Committee. Relevant records of non-paediatric inpatients admitted for snake bite during June 2013 to September 2013 (since introduction of the modified protocol) and June 2012 to September 2012 (i.e., similar period before the new protocol was instituted -historical controls) were summarized into a case record form. These records were reviewed to gather data regarding outcome {discharge after recovery/death/ Discharge Against Medical Advice (DAMA)/absconded}; duration of hospital stay; duration of MICU stay (among those admitted to MICU); number of ASV vials required per patient; need for FFP and number of FFP units required (among those given FFP); need for dialysis and number of dialysis required (among those requiring dialysis).
Details of The ASV Protocols
Criteria for ASV administration: Cellulitis and local swelling with or without bite marks; bleeding (spontaneous gastrointestinal bleeding, uncontrolled bleeding from external wounds), hypotension -blood pressure <90/60 mm of Hg, shock requiring inotropic support; acute kidney injury (oliguria, anuria, and high serum creatinine on admission or rising serum creatinine); in-coagulable blood measured by any of the methods (prolonged clotting time >20 minutes, prothrombin time -international nationalized ratio >1.5, activated partial thromboplastin time >2× control); visible neurological signs.
The ‘conventional protocol’ of ASV was as per Indian guidelines for ASV therapy [17], whereby vasculotoxic snake bite cases received 10 vials ASV stat followed by 5-10 vials repeated at six hourly intervals till normalization of coagulation status or persistence of any sign or symptom (e.g., swelling, respiratory insufficiency, renal failure).
Neurotoxic snake bite cases received 10 vials ASV stat followed by a single repeat dose of 10 vials of ASV, two hours after first dose of ASV, if there was no improvement.
The ‘modified protocol’ for vasculotoxic snake bites was five vials ASV stat followed by two vials repeated six hourly till normalization of Whole Blood Clotting Time (WBCT) to less than 20 minutes. In case of frank bleeding, the dose was 10 vials of ASV stat and five vials at two hourly intervals if bleeding continued. If frank bleeding occurred after the first dose, repeat dose was five vials at six hourly intervals in place of two vials, till bleeding stopped. If patient presented with severe cellulitis the dose was five vials stat. Two vials ASV was repeated six hourly only if cellulitis was spreading.
ASV dosage for neurotoxic snake bites was five ASV vials stat followed by five vials ASV repeated once after two hours, if symptoms were unimproved or progressing. Patients showing no response to neostigmine or requiring ventilator support were administered 10 vials stat and 10 vials after two hours. No neurotoxic snake bite patient received more than 20 vials in total.
Statistical Analysis
Quantitative data between the two groups was compared using unpaired t-test while qualitative data was compared using Chi-square test and Fischer’s- Exact test. Poisonous snake bite cases that absconded or took discharge against medical advice were excluded from the primary analysis and only those cases that completed treatment up to definitive outcome of recovery or death were considered for the primary analysis for outcome, hospital stay, FFP requirement, need for dialysis and number of ASV vials. Secondary analysis was also done using all cases of poisonous snake bite irrespective of outcome.
Results
A total of 247 snake bite cases were admitted to the hospital during June 2012 to September 2012, of which 45.75% were due to poisonous snakes while during June 2013 to September 2013, there were 217 snake bite admissions of which 45.16% were due to poisonous snakes [Table/Fig-1].
Types of snake-bite and outcome of poisonous snakebite cases.
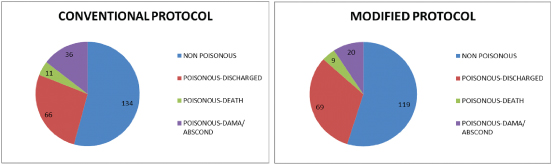
Mean age was 39.26±1.73 years vs 36.19±1.78 years respectively and proportion of males (59.74% vs 60.26%) was similar in both conventional and modified protocol groups. Average ASV requirement showed significant reduction from 28.17±2.75 vials with conventional protocol to 10.74±0.95 vials with use of the modified protocol (p<0.001). The subgroup of patients who were discharged received 29.46±2.92 vials in conventional group and 10.29±0.95 vials in modified protocol group (p<0.001). In case of those who absconded or went DAMA before complete recovery, the requirement was 26.47±2.52 vials vs 9.4±1.15 vials (p<0.001) and in case of patients who died, it was 20.36±8 vials vs 14.22±3.87 vials under the conventional and modified protocols respectively. Nearly 23 patients required more than 30 vials under the conventional protocol while under the modified protocol, only three patients required more than 30 vials of ASV. The maximum ASV requirement per patient was 106 vials under the conventional protocol, while it was just 49 vials under the modified protocol. Despite lower dose of ASV, the mortality (11 deaths/113 cases vs 9 deaths /98 cases) [Table/Fig-1] as well as hospital stay, need for FFP per patient (p=0.051) and dialysis per patient (p<0.01) were not adversely affected by the modified protocol [Table/Fig-2].
Primary analysis: Comparison of conventional and modified protocols on patient management parameters (Mean± SE).
| Parameter | Conventional protocolN=77 | Modified protocolN=78 |
|---|
| Age (years) | 39.26±1.73 | 36.19±1.78 |
| Sex (M:F) (number) | 46:31 | 47:31 |
| ASV vials | 28.17±2.75 | 10.74±0.95*** |
| Number of patients requiring FFP | 18 | 12 |
| FFP Units (bags) | 7.33±1.51 | 4.08±0.43 |
| Number of patients requiring dialysis | 2 | 5 |
| No. of times-dialysis | 5.00±0.00 | 2.4±0.4** |
| Duration of stay in hospital (days) | 4.81±0.35 | 4.88±0.36 |
| No. of patients treated in MICU | 15 | 31** |
| Duration of stay in MICU (days) | 3.8±0.65 | 3.29±0.43 |
***p<0.001 Modified protocol vs conventional protocol
** p<-0.01 Modified protocol vs conventional protocol
The primary analysis considered only those subjects in whom outcome was known i.e., recovery or death. Patients who went home against medical advice or absconded were excluded because, in these cases, the true total ASV requirement and other parameters were modified by the premature discharge from the hospital. Additionally, secondary analyses was done considering all subjects (including DAMA and abscond cases) irrespective of outcome. The average ASV requirement was 27.63±2.03 vials in conventional protocol and 10.47±0.79 vials in modified protocol group (p<0.001). FFP transfusion was required for 24 cases (mean 7.33±1.21) in conventional protocol and in 15 patients from modified protocol (mean 3.93±0.31, p<0.05) while dialysis was required for 3 patients (mean 5 cycles) in the conventional protocol group and 5 patients (mean 2.4±0.4 cycles, p<0.01) in modified protocol group [data not shown in table].
Amongst those receiving conventional doses of ASV, 15 patients were admitted to ICU while 31 from the modified protocol group required ICU stay (p<0.01). Therefore, for statistical comparison, the subjects from both groups were stratified according to need for ICU stay and comparisons were made between the 2 protocols as well as between ICU vs non-ICU patients within a given protocol. Average requirement of ASV in non-ICU cases of modified protocol was lower compared to the ICU cases of modified protocol (p<0.05) as well as the non-ICU cases of the conventional protocol (p<0.001) [Table/Fig-3]. When ICU cases were compared with non-ICU cases, the conventional protocol group had similar duration of hospitalisation (p=0.13) while in the modified protocol group, the stay was significantly shorter in the non-ICU cases (p<0.01) [Table/Fig-3]. The proportion of deaths among ICU cases of conventional protocol were higher than those in the ICU cases of modified protocol (p<0.05) as well as in comparison to the non-ICU cases of the conventional protocol (p<0.001).
Comparison of Conventional protocol and Modified protocol based on requirement for ICU admission (Mean±SE).
| Parameter | Conventional protocolN=77 | Modified protocolN=78 |
|---|
| ICU casesn=15 | Non-ICU casesn=62 | ICU casesn=31 | Non-ICU casesn=47 |
|---|
| No. of ASV vials | 21.53±6.66 | 29.77±3 | 13.45±1.64# | 8.96±1.09*** |
| Duration of Hospitalisation (days) | 6.27±1.10 | 4.45±0.33 | 6.23±0.75## | 4.00±0.27 |
| ICU stay (days) | 3.80±0.65 | - | 3.29±0.43 | - |
| Deaths (n) | 8 | 3@@@ | 6$ | 3 |
***p<0.001 Modified protocol vs conventional protocol for Non-ICU cases
$p<-0.05 Modified protocol vs conventional protocol for ICU cases
#p<-0.05 ICU cases vs Non-ICU cases for Modified protocol
##p<-0.01 ICU cases vs Non-ICU cases for Modified protocol
@@@p<-0.001 ICU cases vs Non-ICU cases for conventional protocol
Additionally, to further evaluate the effectiveness of the modified protocol, we performed a sub analysis based on vasculotoxic, neurotoxic or mixed manifestations at presentation. Of the 78 cases with known outcome in modified protocol group, five had mixed, six had neurotoxic and 67 had vasculotoxic manifestations. The average requirements of ASV were 11.19±3.99 vials for vasculotoxic manifestations and 7.5±1.11 vials for neurotoxic manifestations (p=0.029). The ASV requirement for mixed manifestations was intermediate (8.6±1.50 vials). Mortality was 8 of 67 (11.94%) with vasculotoxic manifestations and 1 of 5 (20%) with mixed manifestations, while there were no deaths (0%) among those with neurotoxic manifestations.
Discussion
In our study we found that, around 45%-46% of snake bite cases have signs of evenomation, with a predominance of signs and symptoms of vasculotoxicity. This proportion is more or less consistent with the reports which found that bites with envenoming constitute 12%-50% of the total number of bites in Asia and 18%-30% in India and Pakistan [2]. When evenomation occurs, the fatal dose of cobra is 120 mg, Russell’s viper is 150 mg, krait 60 mg and Echis carinatusis 80 mg (but venom injected at the time of bite is only 4.6 mg). The amount of venom neutralized by one ml of polyvalent ASV in cobra is 0.6 mg, Russell’s viper 0.6 mg, krait 0.45 and Echis carinatus 0.45 mg, thus, empirically total ASV required is 200 ml, 250 ml, 134 ml and 10.22 ml respectively [18]. However, owing to the growing concern related to shortage and cost of anti-snake venom, several clinicians and researchers have evaluated various low dose ASV protocols for management of snake bite cases. In the present study, we found that our modified protocol resulted in significant reduction in requirement for ASV without any adverse impact on outcome. Similarly, the duration for hospitalization and mortality was similar in both the treatment protocols (11/ 77 in conventional and 9 /78 modified protocol group respectively, p= 0.6398). Furthermore, the patients from modified protocol group required fewer average number of dialysis and relatively fewer units of fresh frozen plasma. A single vial of polyvalent ASV costs around 687.00 INR and the average reduction of around 17.43 vials achieved with the modified protocol results is significant reduction in average cost of ASV by 11974.41 INR per treated patient.
Thomas PP and Jacob J et al., compared a protocol of four ampoules of antivenom in the first hour, four in the next two hours, four in the next three hours, and then three every subsequent three hours to one group with two ampoules of antivenom in the first hour, two in the next two hours, and two every subsequent three hours to another group till clotting time became normal (under 10 minutes) followed by two or three ampoules of antivenom over 24 hours to neutralise any further venom that might be absorbed. They observed that the low dose protocol was as effective as the higher doses [8]. Similarly, Kothari D et al., compared a low dose continuous intravenous regimen (20 ml over two hours followed by 20 ml every 24 hours) with high dose intermittent bolus regimen (30-100 ml over one hour followed by of 30-100 ml every 24 hours) of ASV till correction of coagulation parameters or neurological signs in total 100 patients and reported that low dose ASV had lower mortality and shorter duration for recovery of coagulation parameters and hospital stay as well as the reaction to ASV was not different [9].
Paul V et al., treated snake bite cases with six vials or 12 vials of ASV, irrespective of type of snake or severity of bite and concluded that high dose of ASV did not offer any additional advantage. On the contrary, most of the parameters like mortality, duration of hospital stay and need for dialysis showed a beneficial trend for the low-dose group along with considerable financial gain [10]. One of the possible explanations is that the ASV being made from equine protein may cause subclinical toxic effects in a patient who already has multi-organ involvement, and this may tilt the balance adversely against the patient.
A systematic review of five randomized clinical trials comparing low versus high dose ASV in poisonous snake bite reported that low-dose ASV is equivalent or may be superior to high-dose ASV in management of poisonous snake bite. They also found that low dose is highly cost-effective as compared to the high dose. The doses of ASV in these studies varied in the high dose group from 40 ml to 550 ml, and in the low dose group from 20 ml to 220 ml [11]. Srimannarayana J et al., conducted a prospective interventional study on 90 adult patients with haemotoxic snake bite [12]. They evaluated standard high dose regimen of conventional, intermittent bolus dosage of 100 ml of ASV followed by 50 ml every six hours till whole blood coagulation time became normal against low dose regimen 30 ml or 70 ml of ASV as a loading dose followed by 30 ml continuous infusion every six hours till two coagulation times at an interval of six hours were normal and a further dose of 30 ml over 24 hours and found that lower dose of continuous intravenous ASV infusion helped to reduce ASV requirement and recurrence of coagulation dysfunction [12]. This protocol was somewhat similar to our modified protocol, but this study did not comment on the other complications of snake bite, need for supportive care and length of stay amongst the different regimen.
Tariang DD et al., compared high dose (two vials over one hour, followed by two vials over four hours and repeated four hourly until clotting parameters normalized and then two vials as infusion over 24 hours) with low dose (two vials over one hour, followed by one vial over four hours, repeated four hourly until clotting parameters were normalized and then one vial as an infusion over 24 hours) in a double blind randomised controlled trial. This study found that low dose regimen was cost-effective with no difference in the transfusion, dialysis, ventilation requirement or mortality [13]. Cherian AM et al., conducted a single arm, prospective descriptive study in 54 snakebite patients who were initially given two vials of ASV followed later with one vial at a time according to clotting time along with necessary supportive measures and reported that occurrence of Acute Renal Failure (ARF), neuropraralysis severe enough to require ventilatory support and mortality were similar to that reported with higher doses of ASV in literature [14].
Agarwal R et al., evaluated the effects of two different dosage protocols {(100 ml ASV followed by either 100 ml) six hourly until recovery of neurological manifestations (high dose) or 100 ml of ASV at presentation followed by 50 ml six hours (low dose)}, on the outcome of patients with severe neurotoxic snake envenoming, retrospectively and concluded that there was no difference between the two protocols with respect to duration of mechanical ventilation and duration of intensive care unit. Rather mortality was apparently lower in the low dose group [15].
Gadwalkar SR et al., performed a retrospective analysis in 155 snakebite patients ≥15 years with signs and symptoms of local as well as systemic envenomation. For analysis, patients were stratified as low dose ASV group (received <10 vials) and high dose ASV group (received ≥10 vials) and it was found that high dose and low-dose group showed similar efficacy with respect to neuroparalysis requiring ventilator support and mortality rate. This study analysed the data of patients that inadvertently received less or more than 10 vials, but the study defines no protocol to be followed for low dose, thus practical application of this finding is apparently poor [16].
In view of the findings of our study as well as the other reports, it appears that low dose ASV is an effective and economical mode of management of snake bite cases. High doses of ASV, apart from being non-superior and more costly, may at times turn out to be more harmful as reported by Paul V et al., [10]. In this trial, the mortality rate and the percentage of cases requiring dialysis was more in the high dose group.
An important observation in the present study was that relatively more number of subjects from the modified protocol group were hospitalized in MICU. However, this was a result of purposeful admission to MICU even for less critical cases (as a safety measure) as the dose of ASV planned for use was lesser than that as per the conventional protocol of the hospital. Analysis after stratification by ICU admission and protocol revealed that in the modified protocol group, the ICU cases required more vials of ASV per patient than the non-ICU cases and this figure was similar to the number of vials required under the conventional protocol. Also, patients from the modified dose protocol admitted to the ICU required longer hospitalization than non-ICU cases. These findings actually reflect the makeshift rescue measure incorporated in our modified protocol to administer higher doses of ASV for cases that present with frank bleeding or neurotoxic snake bite showing poor response to neostigmine. Additionally, though the quantity of ASV per patient, among ICU cases, was not different between both the protocols, the mortality was significantly lower in the modified protocol group. Thus, with this rescue measure it was possible to economize on the snake bite treatment, with higher doses used for severe cases, and better outcomes in the ICU cases.
Though there are several studies comparing different doses of ASV, our study has the distinction and additional strength of incorporating a back-up rescue protocol of giving additional high doses in a need based manner depending on patients’ response. This kind of protocol is not only cost-effective but allows a quick switch to the conventional high dose protocol in non-responsive cases and ensures involvement of senior and experienced physicians in patient care for better outcomes.
Limitation
As the study was retrospective in nature, the likelihood of certain factors confounding the findings cannot be overlooked. Also, this study did not include paediatric population, so the clinical implications of using the modified protocol in the paediatric age group cannot be extrapolated. Nevertheless, considering the findings of our study and the lack of consensus statement on minimum recommended dose of ASV, it would be worthwhile to study the modified protocol, which uses lower dose of ASV, in a larger randomized controlled setup.
Conclusion
The fear and risk of death or complications associated with the snake bite, though real, are not as grave as it is perceived. Lower doses of ASV are as effective as the conventional high dose without being associated with higher mortality or morbidity. Judicious use of ASV with rescue protocols at hand can help to save funds as well as more lives with available resources. Whether it is possible to use still lower doses of ASV remains to be studied and should be evaluated for efficient use of health care resources.
***p<0.001 Modified protocol vs conventional protocol
** p<-0.01 Modified protocol vs conventional protocol
***p<0.001 Modified protocol vs conventional protocol for Non-ICU cases
$p<-0.05 Modified protocol vs conventional protocol for ICU cases
#p<-0.05 ICU cases vs Non-ICU cases for Modified protocol
##p<-0.01 ICU cases vs Non-ICU cases for Modified protocol
@@@p<-0.001 ICU cases vs Non-ICU cases for conventional protocol