There are very few reports of invasive infections caused by this fungus from India [13,14,17], which could be attributed partly to the lack of awareness among clinicians and microbiologists and partly to the lack of facilities for culture and accurate identification of the yeast. We recently published a case report of pneumonia caused by S. capitata in a post renal transplant patient from our centre [17]. Realising the paucity of information from India regarding the infections caused by this rare yeast, we decided to review all the patients admitted in our hospital over a period of 10 years (from 2007 to 2016), from whom S. capitata was isolated, so as to better understand the epidemiological, clinical and microbiological characteristics of infections caused by this yeast.
Materials and Methods
A retrospective review of all the patients from whom S. capitata was isolated over the last 10 years (January 2007 to December 2016) in a 1000 bedded tertiary care, postgraduate teaching hospital, located in the city of Hyderabad, Telangana state, Southern India was done. A review of the Microbiology laboratory records of the last 10 years identified a total of six patients with isolation of Saprochaete capitata from blood and other clinical samples. The epidemiological (age, sex, underlying disease and risk factors), clinical, imaging and microbiological data of these six patients were collected and analysed.
Patient 1
A 48-year-old female presented to our hospital in March 2007 with complaints of fever, shortness of breath and generalized weakness since one month. She was evaluated prior to admission, at a local hospital and was diagnosed with biphenotypic acute leukaemia and was referred to our hospital for further management. On day one of admission, her complete blood test revealed a total leukocyte count of 16,100/mm3 with 98% blasts and 2% Neutrophils (ANC 320/mm3) along with thrombocytopenia. Induction chemotherapy with Daunorubicin and Cytosar (7+3 regimen) was started on day 4 post admission. On day 8 (fifth day after initiation of chemotherapy), total leukocyte count reduced to 800/mm3 with differential count showing blasts cells predominantly with only 6% neutrophils (ANC-48/mm3). ANC remained consistently below 50, throughout the hospital course. On day 11, patient developed fever, abdominal pain and diarrhoea. She also complained of haematuria and passage of blood in stools which was attributed to thrombocytopenia. She was started on empiric therapy with Injection Imipenem 500 mg IV 6th hourly but the patient showed no clinical improvement. As per the standard hospital protocol, two sets of blood cultures {each set consisting of one BacT/Alert-FA/3D and one BacT/Alert SA/3D bottles (bioMérieux, Marcy l’Étoile, France)} were done on day 16 and empiric antifungal therapy started on the same day with IV Amphotericin B emulsion 320 mg/day (5 mg/kg bodyweight/day). Both sets of blood cultures were detected positive with a mean Time To Detection (TTD) of 31.7 hours in the BacT/Alert system. The bottles were subcultured on Sabouraud Dextrose Agar and the growth was subsequently identified as Saprochaete capitata. The details of blood culture isolation are summarized in [Table/Fig-1].
Details of positive blood cultures from patient 1.
| S. No. | Day after admission | No. of sets sent | No. of sets showing growth | Mean TTD* | Organism isolated |
|---|
| 1 | Day 16 | 2 | 2 | 31.7 hours | S. capitata |
| 2 | Day 18 | 2 | 2 | 14.75 hours | S. capitata from both the sets along with E. gallinarum |
| 3 | Day 19 | 2 | 2 | 24.6 hours | S. capitata and E. gallinarum |
| 4 | Day 20 | 2 | 2 | 23.2 hours | S. capitata |
| 5 | Day 21 | 2 | 2 | 22.8 hours | S. capitata |
*TTD-Time To Detection
As blood cultures sent on 18th and 19th day also showed growth of Enterococcus species, Injection Vancomycin was added to Amphotercin B (AMB) which the patient was already receiving. The Enterocccus spp. was later identified as E. gallinarum using the Vitek II GP ID card (bioMérieux, Marcy l’Étoile, France). Patient developed multi organ dysfunction with hepatopathy and Acute Respiratory Distress Syndrome (ARDS) and succumbed to fungal sepsis on day 22 post admission.
Patient 2
A 65-year-old female patient presented to the outpatient department in September 2007 with complaints of fever, shortness of breath, decrease in appetite and loss of weight, all since one month. Patient also noticed a lump in the abdomen about 20 days back. On examination patient was found to have significant pallor, hepatosplenomegaly, bilateral rhonchi and crepitations in the lung. Complete blood test showed haemoglobin 7.2 g/dl, total leukocyte count 511,000/mm3 and platelets 60,000/mm3. A total of 95% of the leukocytes were small lymphoid cells with scanty cytoplasm and numerous smudge cells with only 3% neutrophils. Bone marrow examination confirmed the diagnosis of chronic lymphocytic leukaemia. She was admitted and received chemotherapy (cycle1) with Fludarabine and Cyclophosphamide from day 2 to day 4 post admission. Patient was also on prophylaxis with oral fluconazole 100 mg, once daily and co-trimoxazole, one double strength tablet (160/800 mg) on alternate days. On day 5, patient developed a spike in fever and blood cultures as per the standard hospital protocol were sent the next day. After sending blood cultures, patient was started empirically on Injection Cefoperazone/sulbactam and Amikacin. One of the four blood culture bottles sent on day six showed growth of an yeast (TTD-20 hours) identified later as S. capitata using the mini API (bioMérieux, Marcy l’Étoile, France) ID 32C strip, while the other three bottles grew coagulase negative staphylococci. No specific antifungal therapy was started as significance of the isolates was uncertain. Patient became asymptomatic within 48 hours of starting the antibacterial therapy, which was continued for 8 days. Patient was discharged in a stable condition on day 17. Subsequently patient received three more cycles of chemotherapy and was on medical oncology follow up till four months after discharge.
Patient 3
A 19-year-old female patient was admitted in January 2008 to our hospital with complaints of fever associated with gum bleeding and vaginal bleeding, since three days. On examination, patient was febrile and had significant pallor. Complete blood test on day 1 showed haemoglobin 6.3 g/dl, total leukocyte count 900/mm3 with ANC of 360/mm3 and platelets 10,000/mm3. Empiric antimicrobial therapy with Cefoperazone/sulbactam, amikacin and metronidazole was started. On day 2 bone marrow examination was done, which showed a picture of acute myeloid leukaemia. Chemotherapy was deferred because of ongoing febrile neutropenia. On day 3, there was no clinical improvement and patient complained of dry cough, nasal block, epistaxis, yellowish discolouration of the eyes. Cefoperazone/sulbactam and amikacin were stopped and the patient was started on IV meropenem. Blood cultures were sent on day 4 post admission of which one bottle showed growth of yeast the next day while the other three bottles did not show any growth. The yeast was identified as Candida tropicalis using the mini API (bioMérieux, Marcy l’Étoile, France) ID 32C strip. On day 6, the patient was enrolled in an ongoing Phase III, double- blind, randomized study to evaluate safety and efficacy of Isavuconazole versus Caspofungin in the treatment of Candidemia and other invasive Candida Infections. Blood cultures were repeated on day 7. On day 9, patient complained of worsening cough with haemoptysis, shortness of breath and on examination showed fine crepitations in the lung bilaterally in the inframammary regions. Complete blood picture on day 9 revealed a total leukocyte count of 400/mm3 with ANC of 40/mm3. Blood cultures sent on day 7 were reported on day 9 to grow a non-Candida yeast possibly Trichosporon/Saprochaete species in both the sets. The yeast was subsequently identified as S. capitata. The trial drug was discontinued and the patient was started on an IV infusion of Amphotericin B, 50 mg/day on day 10. Repeat blood cultures also showed the growth of S. capitata. Details of blood culture isolation are summarized in [Table/Fig-2].
Details of positive blood cultures from patient 3.
| S. No. | Day after admission | No. of blood culture sets | No. of bottles/sets showing growth | Mean TTD* | Organism isolated |
|---|
| 1 | Day 4 | 2 | Only one bottle of the 2 sets | 22.3 hours | Candida tropicalis |
| 2 | Day 7 | 2 | 2 | 27.6 hours | S. capitata |
| 3 | Day 8 | 2 | 2 | 29.5 hours | S. capitata |
| 4 | Day 9 | 2 | 2 | 19.7 hours | S. capitata |
| 5 | Day 10* | 2 | 2 | 14.7 hours | S. capitata |
#TTD-Time To Detection
*Sputum culture done on the same day also showed the growth of S. capitata.
Sputum sent for fungal culture on day 10 also grew the yeast. Patient developed multi organ dysfunction with ARDS and hepatic failure and succumbed on day 12 after admission.
Patient 4
A 60-year-old male, known case of diabetes mellitus Type II and hypertension presented to our centre in June 2013 with complaints of fever, cough with haemoptysis, Grade III dyspnoea and right sided chest pain since two weeks. His chest pain increased on inspiration and coughing. Patient was initially admitted to a local hospital where the chest X-ray showed collapse of the lung with pleural effusion on the right side. Ultrasound chest showed right side massive pleural effusion with multiple thick internal septations and underlying collapsed lung. Left pleural space was clear. Inter Costal Drain (ICD) was placed on the right side and the patient was initiated on empiric anti tubercular therapy. Patient was then referred for further management to our centre. On day 1 of admission, complete blood test showed haemoglobin 9.5 g/dl, total leukocyte count 47,400/mm3 with 88% neutrophils. US guided aspiration of pleural fluid was done on day 2, day 5 and day 9. The pleural fluid analysis showed elevated leukocyte counts 4800/mm3 with 80% neutrophils, low glucose and elevated adenosine deaminase levels (84.7 IU/ml). Bacterial, mycobacterial and fungal cultures were all negative. As the Computed Tomography (CT) scan done on day 8 showed persisting pockets of empyema with thick septations not amenable to aspiration, pulmonectomy was advised. After starting anti tubercular therapy, patient showed clinical improvement with decrease in cough and dyspnoea but continued to have low grade fever. Complete blood test on day 15 showed Haemoglobin (Hb) 9.5 mg/dl, total leukocyte count of 8600/mm3 with 54% neutrophils and 31% lymphocytes. Surgery was deferred and patient was discharged on day 16 with advice to continue anti tubercular therapy and come for follow up in the outpatient department. Out of the two sets (four bottles) of blood cultures sent the day before discharge (day 15), three bottles showed no growth while one bottle flagged positive (TTD- 3.36 days) for yeast, which was identified as Saprochaete capitata. The patient did not come back for follow up.
Patient 5
A 58-year-old female patient, presented with intermittent high grade fever with chills and rigors, dysuria, yellowish discolouration of the eyes, nausea, vomiting and decreased appetite since one month. Patient was a known case of diabetes mellitus Type II on treatment with oral hypoglycaemic agents since eight years. On examination patient was conscious, coherent and had icterus. Investigations done before admission showed total bilirubin 7.3 mg/dl with conjugated bilirubin 4.6 mg/dl, Aspartate Amino Transferase (AST)-86 U/L, Alanine Amino Transferase (ALT) 80 U/L, Alkaline Phosphatase 348 U/L, total protein 6.4 g/dl, albumin 2.6 g/dl and Serum creatinine 4.1 mg/dl. Urine examination showed 10-12 pus cells/HPF and was positive for sugar and albumin. Patient’s blood was positive for Hepatitis B surface antigen and Hepatitis C Virus (HCV) antibodies while Enzyme Linked Immuno Sorbent Assay (ELISA) for Human Immunodeficiency Virus (HIV) antibodies was negative. Complete blood test on day 1 of admission showed Hb 11.5 g/dl, total leukocyte count 9100/mm3 with 69% neutrophils, 37% lymphocytes and platelet count 150,000/mm3. Blood and urine were sent for culture and sensitivity and patient was started empirically on Inj Piperacillin/tazobactam. Urine culture and sensitivity was reported on day 2 as showing significant growth of Extended Spectrum Beta Lactamase (ESBL) producing strain of Escherichia coli. All other investigations like chest X-ray, 2D Echocardiography (2D ECHO), ultrasound abdomen were normal. As per the culture and sensitivity report, patient was started on Injection Meropenem on day 3. Patient’s fever and dysuria completely subsided by day 6 and serum creatinine decreased to 1.9 mg/dl. Two sets of blood cultures sent on day 1 of admission did not show any microbial growth after 48 hours of incubation but on further incubation one of the four bottles was reported positive for yeast (TTD-3.43 days) on day 5 of incubation. As patient recovered completely with IV antibiotics given for treatment of Urinary Tract Infection (UTI), no antifungal therapy was initiated. The yeast was identified as S. capitata on day 7 and on day 8 patient was discharged in a stable condition with advice to be on follow up for her multiple comorbidities. The patient was lost to follow up.
Patient 6
A 35-year-old male was admitted with fever and productive cough of 20 days duration. Fever was high grade and intermittent. It was associated with copious yellowish coloured sputum, which was foul-smelling. He had undergone a live related renal transplant, with sister as a donor four years ago. He did not receive any induction therapy and at presentation, he was on triple immunosuppression with tacrolimus 0.5 mg twice daily, mycophenolate mofetil sodium 360 mg thrice daily, and prednisolone 5 mg once daily. X-ray chest showed consolidation of right lower lobe. High resolution CT of the chest showed consolidation and nodular lesions in the lateral basal segment of right lower lobe. Bronchoalveolar lavage was performed and culture from this fluid grew yeast which was identified as Saprochaete capitata sensitive to amphotericin, voriconazole, and flucytosine. The patient was started on conventional amphotericin B at a dose of 1 mg/kg/day along with oral voriconazole, loading dose 400 mg/twice a day on day 1, followed by a maintenance dose of 200 mg/twice a day. After 10 days, patient’s fever and cough subsided completely and repeat culture of Broncho Alveolar Lavage (BAL) did not show growth of any yeast. Amphotericin was discontinued after two weeks and voriconazole was continued for a total of six weeks. This case has been published earlier by us as a individual case report [17], but has been included in the present study as the purpose was to review all patients with isolation of S. capitata from our centre till December 2016.
The epidemiological, clinical and microbiological data of these 6 patients from our centre have been summarized in [Table/Fig-3].
Epidemiological, clinical and microbiological data of the 6 patients reviewed.
| S. No. | Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|
| 1 | Age/Sex | 48/F | 65/F | 19/F | 60/M | 58/F | 35/M |
| 2 | Underlying disease | Biphenotypic acute leukaemia | Chronic lymphocytic leukaemia | Acute myeloid leukaemia | DM type II, HTN | DM type II, found to be HbS Ag and HCV positive after admission | Post renal transplant |
| 3 | Chemotherapy | Induction | Induction | Not started because of ongoing febrile neutropenia | NA | NA | Immunosuppression with tacrolimus, prednisolone and mycophenolate mofetil |
| 4 | Duration of Neutropenia (<500/cu.mm) before isolation of S. capitata | 9 days | 6 days | 7 days | NA | NA | No neutropenia |
| 5 | Day post initiation of chemotherapy when S. capitata was isolated | 13 days | 5 days | NA | NA | NA | NA |
| 6 | Clinical manifestations | Fever, abdominal pain, diarrhoea, hepatopathy, ARDS, septic shock | Fever, shortness of breath, bilateral crepitations | Fever, cough with haemoptysis, shortness of breath, nasal block, crepitations bilaterally in the infra mammary areas | Fever, cough with mucoid expectoration, haemoptysis, shortness of breath, right sided Chest pain. Chest X-ray and CT-masssive pleural effusion with thick internal septations and underlying collapsed lung. | Fever associated with chills and rigor, dysuria. Urine culture showed significant growth of ESBL producing E.Coli | Fever, productive cough since 20 days. HRCT chest showed consolidation and nodular lesions in the lateral basal segment of the right lower lobe of lung |
| 7 | Sample from which S .capitata was isolated | Blood | Blood | Blood and sputum | Blood | Blood | Bronchoalveolar lavage |
| 8 | No. of positive blood cultures | 5 | 1 | 5 | 1 | 1 | None |
| 9 | Concomitant organisms isolated | Enterococcus gallinarum | Coagulase negative staphylococci skin contaminant | None | None | None | None |
| 10 | Antifungal therapy | AMB emulsion(320 mg/day/IV)-5 mg/kg body weight | No specific antifungal therapy started as the growth was seen in only one of four blood cultures bottles sent on day 6 of admission, while the rest 3 bottles grew coagulase negative staphylococci. Patient recovered with only broad spectrum antibacterial therapy. | Inj AMB 50 mg IV OD -started on day 10 after admission, after identification of yeast as S. capitata | No specific antifungal therapy started. Patient responded to antitubercular therapy and repeated drainage of pleural fluid. Decortication advised but deferred to a later date. Patient was stable at discharge. | No specific antifungal therapy initiated as only one of four bottles sent on the day of admission grew yeast. Patient became asymptomatic on therapy with Meropenem for UTI. | i) dAMB 1mg/kg body weight-2 weeksii) Oral Voriconazole - 400 mg BD-1 day followed by 200 mg BD-6 weeks |
| 11 | Outcome | Death on day 22 post diagnosis | Recovery-followed up till 4 months. | Death on day 12 | Recovery | Recovery | Recovery |
| 12 | Remarks | Clinically significant | ? Transient Fungemia | Clinically significant | ? Transient Fungemia | ? Transient Fungemia | Clinically significant |
*Diabetes Mellitus Type II and Hypertension (DM type II and HTN), Hepatitis B Surface Antigen and Hepatitis C Virus (HBsAg and HCV), Hepatitis C Virus (HCV), Acute Respiratory Distress Syndrome (ARDS), Amphotericin B (AMB), Extended Spectrum Beta Lactamase (ESBL), High-Resolution Computerized Tomography (HRCT), Urinary Tract Infection (UTI)
Identification and Antifungal Susceptibility of the Yeast Isolates
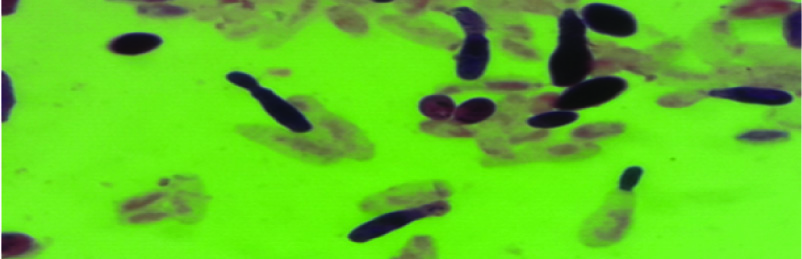
The yeast grew as white to cream coloured colonies on Sabouraud’s Dextrose agar [Table/Fig-4] and blood agar. Microscopic examination of the Lactophenol cotton blue mount from the colony showed anneloconidia [Table/Fig-5], typical of S. capitata along with arthroconidia, blastocondia, true hyphae and pseudohyphae, distinguishing the yeast from the closely related Trichosporon and Geotrichum species. The identification was confirmed by biochemical testing using the mini API (bioMérieux, Marcy l’Étoile, France)- ID 32C strip for isolates 1 to 5 and using the Vitek II (bioMérieux, Marcy l’Étoile, France)-ID Yeast card for the isolate from patient 6. Antifungal susceptibility testing was done using the mini API- ATB Fungus strip (isolates 1 to 5) and for the isolate from patient 6 using Vitek II-YS07 card.
Macroscopic morphology: 48 hour growth of S. capitata on Sabouraud’s Dextrose agar with chloramphenicol (30°C) – white to cream coloured, dry, wrinkled colonies with radiate margins.

Microscopic morphology: Gram stained smear showing annelloconidia of Saprochaete capitata (1000 X).
Results
Saprochaete capitata was isolated from clinical samples in a total of six patients over the last 10 years (2007 to 2016). The age of the patients ranged from 19 years to 65 years with a median age of 53 years. There were two males (patient no. 4 and 6) and four females (patient no. 1-3, 5). The distribution of the different characteristics among these 6 patients is summarized in [Table/Fig-6].
Characteristics of the 6 patients with isolation of S. capitata from clinical samples.
| S. No | Characterstic | No. of patients |
|---|
| 1 | Underlying diseases
Haematological Acute Leukaemia (Bi Phenotypic) CLL AML
Non haematological Post Renal Transplant DM type II DM type II, Hepatitis B, HCV
| 111111 |
| 2 | Immunosuppressive agents
Cytosar and Daunorubicin Fludarable and Cyclophosphamide Tacrolimus, Mycophenolate mofetil and Prednislone
| 111 |
| 3 | Neutropenia (ANC≤500/mm3) at time of Isolation of S. capitata (Duration of neutropenia before isolation) | 3 (6-9 days) |
| 4 | Specimen from which isolated
Blood Blood and Sputum BAL
| 411 |
| 5 | Isolation S. capitata from clinical samples more than once | 3 |
| 6 | Antifungal use before isolation of S. capitata Oral Fluconazole | 1 |
| 7 | Isolation considered clinically significant (S. capitata isolated repeatedly with no alternative explanation for the clinical presentation | 3 |
| 8 | Antifungal therapy received (duration and patient outcome)
Amphotericin B emulsion IV Amphotericin B deoxycholate IV Amphotericin B deoxycholate + Oral Voriconazole
| 1 (6 days, Death)1 (2 days, Death)1 (2 weeks + 4 weeks, recovered) |
Chronic Lymphocytic Leukaemia (CLL), Acute Myeloid Leukaemia (AML), Diabetes Mellitus (DM), Hepatitis C Virus (HCV), Broncho Alveolar Lavage (BAL)
In three of the six patients (patient no. 1, 3 and 6), the isolation of S. capitata was considered clinically significant as the yeast was isolated repeatedly from blood and or respiratory specimens and the clinical features could not be explained by any other alternative diagnosis. The time to detection of the first positive blood culture in the bacTAlert automated blood culture system was very short ranging from as low as 22.8 hours to 33 hours with a median of 31.25 hours. Patient 1 had features of sepsis with hepatopathy and ARDS, patients 3 and 6 had features of pulmonary infection with cough and haemoptysis with patient 3 having sepsis in addition.
In the other three patients (patient no. 2,4 and 5), the isolation was considered to be of doubtful significance, as the organism was isolated only from one of the four blood culture bottles while the other three bottles either showed growth of probable skin contaminants (patient 2) or showed no growth (patient no. 4 and 5). Except in case of patient 2 where the TTD was 20 hours, the time to detection of growth in BacT Alert blood culture system was long (3.3 days in patient 4 and 3.43 days in patient 5). In these three patients, the clinical presentation could be explained by other coexisting bacterial or mycobacterial infections and all the three patients clinically improved without specific antifungal therapy; patient 2 had signs of pulmonary infection which responded to broad spectrum antibiotics, patient 4 had pulmonary and pleural involvement with clinical improvement in response to anti-tubercular therapy while case 5 had clinical features suggestive of UTI which was successfully treated with antibiotics.
The Minimum Inhibitory Concentrations (MICs) of Amphotericin B and Voriconazole were low for all the six isolates while Echinocandins (Caspofungin and Micafungin) showed high MICs towards the single isolate (from patient 6) which was tested; the mini API ATB fungus 3 strip which was used for Anti Fungal Susceptibility Testing (AFST) of the earlier isolates did not include echinocandins. MICs of Fluconazole and Itraconazole varied from ≤1.00 mcg/ml to 8 mcg/ml and ≤0.125 mcg/ml to 0.500 mcg/ml respectively. The results of Antifungal Susceptibility Testing are shown in [Table/Fig-7].
Antifungal Susceptibility profile of S. capitata isolates.
| Anti fungal agent | MIC in mcg/ml* |
|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|
| Amphoteric in B | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Fluconazole | 1.000 | 1.000 | 1.000 | 8 | 1.000 | 2 |
| Itraconazole | 0.500 | 0.125 | 0.500 | 0.125 | 0.125 | NT |
| Voriconazole | 0.063 | 0.063 | 0.063 | 0.063 | 0.063 | 0.125 |
| Caspofungin | NT | NT | NT | NT | NT | 4 |
| Micafungin | NT | NT | NT | NT | NT | 4 |
*Antifungal susceptibility was done for Isolates 1-5 using ATB fungus strip and read in mini API. biomerieux, France and for isolate 6 using the Vitek 2-YST 07 card (biomerieux, France).
≠NT-not tested.
Clinical and Laboratory Standards Institute (CLSI) or The European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints are not yet available for S. capitata.
Patient 1 received Amphotercin B emulsion and patient 3 received conventional AMB but both patients succumbed to fungal sepsis within a short period of starting antifungal therapy. Patient 6 with pulmonary infection was treated with a combination of AMB deoxycholate and Voriconazole and showed complete resolution of symptoms within 10 days. AMB was stopped after two weeks while Voriconazole was continued for six weeks in this patient.
Discussion
Rare yeasts other than Candida are being increasingly reported as a cause of opportunistic infections in immunocompromised patients [5,6].
Following the first report of systemic infection with S. capitata in 1964 [18] opportunistic infections due to this yeast are being increasingly reported from across the globe. Most cases have been reported initially from Mediterranean region and central Europe [3,4,12,19].
Mazzocato S et al., identified a total of 104 cases of systemic infections due to S. capitata described between 1977 and 2013 [1]. Fifty six percent of the patients were male and the median age was 56 years (range 0.5–76 years), the age and sex distribution being very similar to the present study. Haematological malignancies were found to be most common comorbidities being present in 87% of the cases; Acute Myeloid Lekaemia (AML) (52 %), Acute Lymphoblastic Leukaemia (ALL) (22 %), other haematological malignancies (13 %). Non-haematological diseases (COPD, diabetes, solid tumours, solid organ transplantation and endocarditis) were present in 9% of the cases while in 4% of the cases there were no reported underlying diseases. Severe neutropenia (<500/mm3) was present in 82% of the patients. In the present study four out of the six patients were immunosuppressed, of whom three (50%) had haematological malignancies along with severe neutropenia (<500/mm3) and one patient had undergone renal transplantation and was on Immunosuppression. In the present study, the duration of neutropenia prior to the isolation of the S. capitata in the three neutropenic patients was 9, 6 and 7 days while in other studies the duration varied from an average of 21.5 days [7] to 7 days [8].
In the review by Mazzocato S et al., blood was the most common sample from which the yeast was isolated (75% of the cases) while in the remaining 25%, it was isolated from other sterile sites (CSF, tissue biopsies, urine, peritoneal fluid) [1]. In 43% of the cases more than one site (brain, lung, liver, spleen, kidney, gut, bone, and bone marrow) was clinically involved. Even in the present study, blood was the most common sample (five of the six patients) from which the yeast was isolated, the other samples being sputum and broncho alveolar lavage. In the present study, Saprochaete capitata was isolated from more than one site (from both blood and sputum) in only patient 3.
There is no clarity regarding optimal therapy for infections caused by S. capitata. In the review by Mazzocato S et al., antifungal treatment was specified in 93 patients (89 %) [1]. Monotherapy was utilized in 42% of treated patients while combination (2-4 drugs) or sequential therapies were utilized in the remaining cases. Amphotericin B was the drug most commonly utilized either in monotherapy or combination regimens (76 individual cases) followed by voriconazole, itraconazole, fluconazole, 5-fluorocytosine, caspofungin, posaconazole and terbinafine. As in the present study, most studies [1,2,4,7-9,20-22] suggest good activity of AMB, Flucytosine and Voriconazole against this fungus, inherent resistance to echinocandins and variable susceptibility to Fluconazole and Itraconazole. Based on the limited in vitro and clinical data, Amphotericin B with or without Flucystosine or a combination of Amphotericin B either with high dose Fluconazole (for susceptible strains) or Voriconazole is recommended by most authors [1,2,4].
Breakthrough infections with S. capitata in patients receiving echinocandins or fluconazole have been reported [8,19,23]. In the present study, at the time of isolatioin of S. capitata, only one of the six patients (patient 2) was on Fluconazole prophylaxis.
Overall, in the study by Mazzocato S et al., crude mortality was 60% (62/104), the only variable that was significantly associated with a positive outcome by multivariate analysis was empirical treatment [1]. Girmenia C et al., in their review of 35 patients with invasive infections due to S. capitata reported a crude mortality of 55.7% [3], while Martino R et al., in their review of 25 cases of Invasive infections due to the yeast in patients suffering from acute leukaemia reported a mortality of 52% [8]. In the present study, three out of the six patients received antifungal therapy, of which only one patient survived while the other two succumbed to fungal sepsis soon after therapy was started.
Review of pubmed data base for reports of isolation of S. capitata from India till date, revealed nine cases [13,14,24-29] excluding the case report published from our centre [17]. The cases are summarized in [Table/Fig-8]. In contrast to cases reported from across the world, where blood was the most common specimen from which the yeast was isolated, only one of these nine patients had isolation of the yeast from blood, sputum being the most common sample yielding growth of the yeast. Inadequacy of the blood culture technique coupled with inaccurate identification of rare pathogens may account for the low number of blood culture isolations reported from India [24].
Summary of the Indian case reports of S. capitata infection.
| S. No. | Authors | Year | Age/Sex | Specimen(s) from which yeast was isolated | Underlying disease | Clinical details | Management | Outcome |
|---|
| 1 | Mathews MS and Sen S [24] | 1998 | 6 months/M | Blood, IV fluids | Post surgical repair of eventeration of the left diaphragm | Sepsis on the 7th post operative period.Blood cultures done on postoperative day 7 and cultures of IV fluids administered to the child showed growth of S. capitata | Antifungal therapy could not be started as growth of yeast reported after death. | Death |
| 2 | Gill PK and Gill JS [14] | 2011 | 70 years/F | Sputum | None | Low grade fever and cough since 6 monthsShortness of breath since 2 months.Crepitations bilaterally in the right Interscapular region.X-ray chest: patchy consolidation in middle and lower zone of lungs | Fluconazole 150 mg daily orally-responded to treatment by 7th day, discharged with advice to continue antifungal for 30 days. | Recovery |
| 3 | Sreeja S et al., [25] | 2011 | 58 years/M | Sputum | Chronic smoker, COPD | Fever, cough, and breathlessness -seven days known patient of COPD chronic smoker-18 years.Chest auscultation-decreased air entry in the left infra scapular and infra-axillary areas with bilateral basal crepitations.Chest X-ray-left lower lobe consolidation with para pneumonic effusion and mild pericardial effusion. | No specific antifungal therapy. Patient died of sepsis and multiorgan failure the same day the positive sputum sample was collected. | Death |
| 4 | Das AK et al., [26] | 2013 | 70 years/F | Sputum | Pulmonary Nocardiosis | Shortness of breath-4 yearsFever-1 month,left lower lobe pneumoniaSputum culture-Nocardia isolated along with S. capitata (colonizer) and Staphylococcus aureus | No specific antifungal therapy given. Patient responded to antibacterial therapy with levofloxacin and amoxicillin/clavulanic acid. | Recovery |
| 5 | Dhevahi E et al., [27] | 2014 | 52 years/M | Sputum | Pulmonary tuberculosis | Fever - 2 monthsproductive cough + shortness of breath-1 monthChest X-ray-extensive homogenous opacities- left lung.Sputum AFB PositiveSputum culture-S. capitata | Both patients treated with Itraconazole100 mg for 5 days along with ATT. Patient improved clinically and discharged on day 6 with advice to continue antifungal for 10 more days and also attend DOT centre for ATT. | Recovery |
| 68 years/M | Sputum | Pulmonary Tuberculosis, COPD | Productive cough, shortness of breath-3 monthChest X-ray-fibrotic changes in the apices-both lungsSputum AFB positive,Sputum culture –S. capitata |
| 6 | Devadas SK et al., [28] | 2015 | 33 years/M | FNAC specimen from subcutaneous swelling | Allogenic HSCT for relapsed Acute myeloid leukaemia | Subcutaneous swelling over the sternum and lower part of the anterior chest wall | Initially received Posaconazole 400 mg twice daily-lesions increased in size after four weeks. Posaconazole was stopped and Injection Voriconazole started at a loading dose 6 mg/Kg body weight followed by 4 mg/kg body weight. There was decrease in size of the lesion by two weeks and complete resolution by 21 weeks. | Recovery |
| 7 | Chauhan S et al., [29] | 2015 | 75 years/M | Sputum | Pulmonary tuberculosis in the past | Fever, breathlessness, epistaxis and malaise.On chest auscultation, bilateral rhonchi and crepitations in intrascapular regionX-ray chest showed patchy consolidation in middle and lower zones. | Fluconazole 150 mg/day started but patient left against Medical advice. | Not known. |
| 8 | Sahu SK et al., [13] | 2016 | 52 years/M | Corneal scrapings | The patient an agriculture worker had a foreign body in the eye | Foreign body in the eye5 days later - patient developed a purulent corneal ulcer | Systemic Liposomal Amphotercin B and Topical Antifungal agent | Improved |
*Chronic Obstructive Pulmonary Disease (COPD), Acid Fast Bacilli (AFB), Directly Observed Therapy (DOT), Anti Tuberculosis Therapy (ATT), Fine Needle Aspiration Cytology (FNAC), Hematopoietic Stem Cell Transplant (HSCT)
Conclusion
The increasing population of immunocompromised hosts has led to rise in the incidence of invasive fungal infections, including those caused by rare opportunistic yeasts such as S. capitata. Inability to distinguish invasive infections caused by S. capitata from those caused by the more common candida species may lead to inappropriate treatment with echinocandins, to which S. capitata is resistant. Familiarity with the epidemiology, clinical and microbiological aspects of S. capitata infections is essential for early recognition and appropriate management of these infections.
Chronic Lymphocytic Leukaemia (CLL), Acute Myeloid Leukaemia (AML), Diabetes Mellitus (DM), Hepatitis C Virus (HCV), Broncho Alveolar Lavage (BAL)
*Antifungal susceptibility was done for Isolates 1-5 using ATB fungus strip and read in mini API. biomerieux, France and for isolate 6 using the Vitek 2-YST 07 card (biomerieux, France).
≠NT-not tested.
*Chronic Obstructive Pulmonary Disease (COPD), Acid Fast Bacilli (AFB), Directly Observed Therapy (DOT), Anti Tuberculosis Therapy (ATT), Fine Needle Aspiration Cytology (FNAC), Hematopoietic Stem Cell Transplant (HSCT)