The liver is involved in the regulation of metabolic pathways and transportation of trace elements including their bioavailability, tissue distribution and toxicity. Trace elements are involved in many enzymatic reactions by inhibiting or activating them, including competition with other elements and metalloproteins for binding sites. They also play a key role in maintenance of vital functions and imbalance in the form of excess or deficiency leading to metabolic disorders. As liver is involved in metabolism of these trace elements, there is a significant correlation with trace element metabolism and disease presence and progression. There is also a correlation between serum trace element concentration and metalloprotein concentration in the body, and imbalances play a significant role in liver disease [2-6].
The role of trace elements in pathogenesis of liver cirrhosis and its complications is still not clearly understood [7], this study was conducted to evaluate trace elements in patients with liver cirrhosis and to assess their association with severity of disease.
Materials and Methods
This observational study was conducted in a prospective way in the Department of Medicine, JSS Hospital, Mysore between October 2013 and October 2015. Local Institutional Ethical Committee approval was sought before commencement of the study. Informed written consent was obtained from all recruited subjects prior to participation. A total of 75 patients of either sex who were admitted under the Department of Medicine, JSS Hospital, Mysore were studied. Patients of either sex with cirrhosis diagnosed on the basis of combination of clinical features of portal hypertension- ascites and/or gastrointestinal varices, blood profile with evidence of thrombocytopenia and/or coagulopathy and radiological imaging demonstrating a small shrunken liver with or without splenomegaly and intra-abdominal varices were included in the study. Patients with age<18 years, fulminant hepatic failure, Wilson’s disease, impaired renal function- creatinine clearance less than 60 ml/min, chronic alcoholics, patient on diuretics and malignant disease were excluded. The patients’ demographics, presenting complaints, past medical history and detailed examination findings were recorded soon after admission.
Severity of liver disease was assessed based on CTP score and patients were grouped into Class A, B and C according to total score of 5-6, 7-9 and 10-15. Fasting blood sample (3-4 ml) was drawn from each subject in plain, EDTA, and PT vials for performing following investigations: urea, creatinine, AST, ALT, ALP, bilirubin, total protein, albumin, cholesterol, triglyceride which was performed on fully automated analyser (Olympus AU 400). Serum zinc, manganese, copper and magnesium were assessed on atomic absorption spectrophotometer (model- AAS4141 by ECIL). Complete haemogram was performed on Five Part XT 1800 I Sysmex Analyser. USG abdomen was performed by a single observer using Philips iU-22 with probe C5-1/ L12-5 and upper GI Endoscopy was done by single observer using Olympus GIF-Q180 scope.
Statistical Analysis
Statistical analysis was done using SPSS version 21.0. Microsoft excel was used for graphical representation. Descriptive statistics was done by calculating mean and standard deviation for continuous variables and proportions for categorical variables. Inferential statistics were done using Chi-square test for categorical variables, one-way Analysis of Variance (ANOVA) for parametric data and Kruskall Wallis test for non-parametric data. Pearson correlation and Spearman’s correlation (non parametric data) was used. A p-value of <0.05 was considered as statistically significant.
Results
A total of 75 patients were enrolled in the study; 68 (90.7%) were males. Fifteen (20.0%) were between 30-39 years of age, 27 (36.0%) patients were between 40-49 years of age, 14 (18.7%) patients were between 50 and 59 years, 12 (16.0%) were between 60-69 years of age and 7 (9.3%) above 70 years of age.
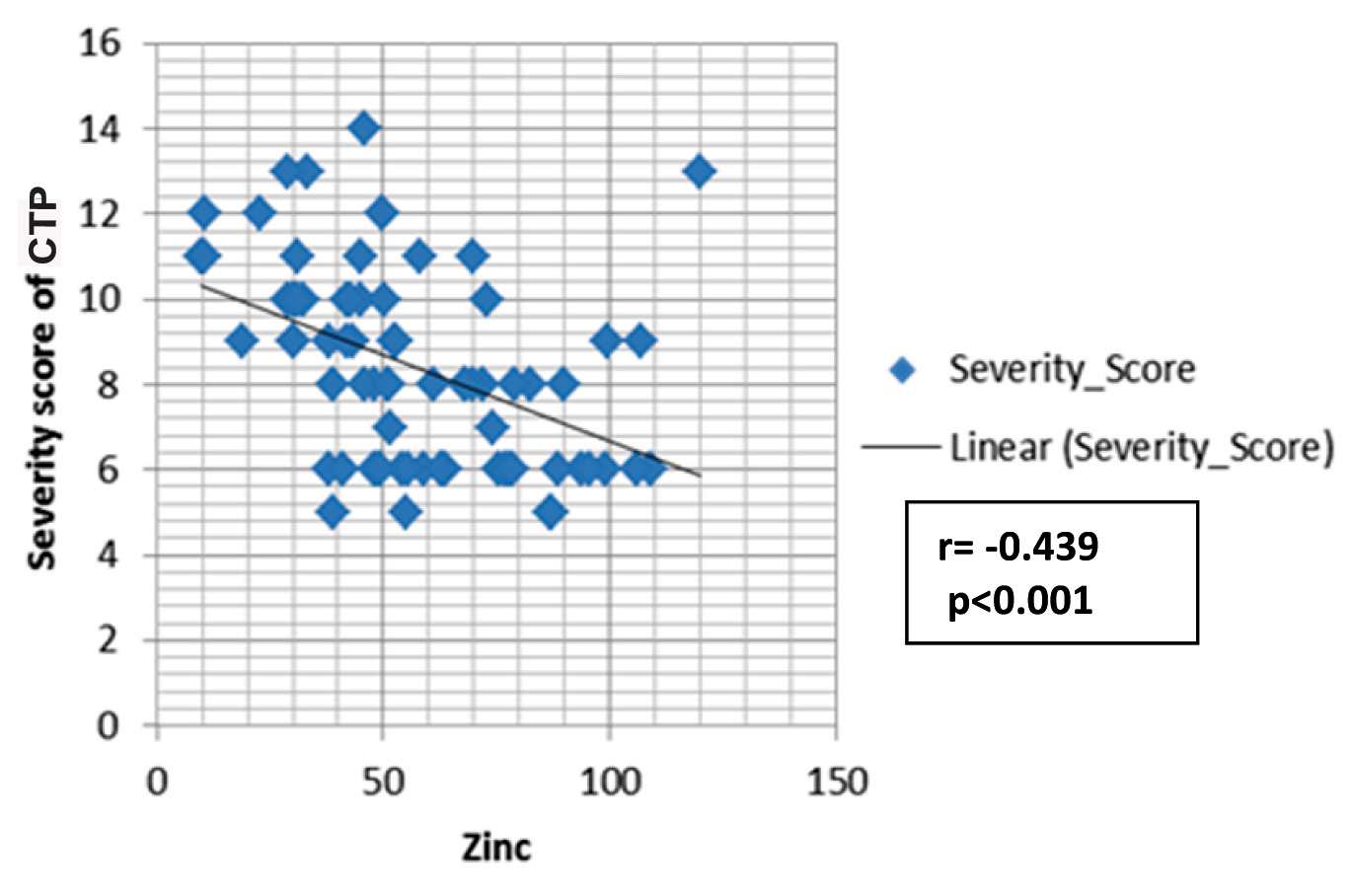
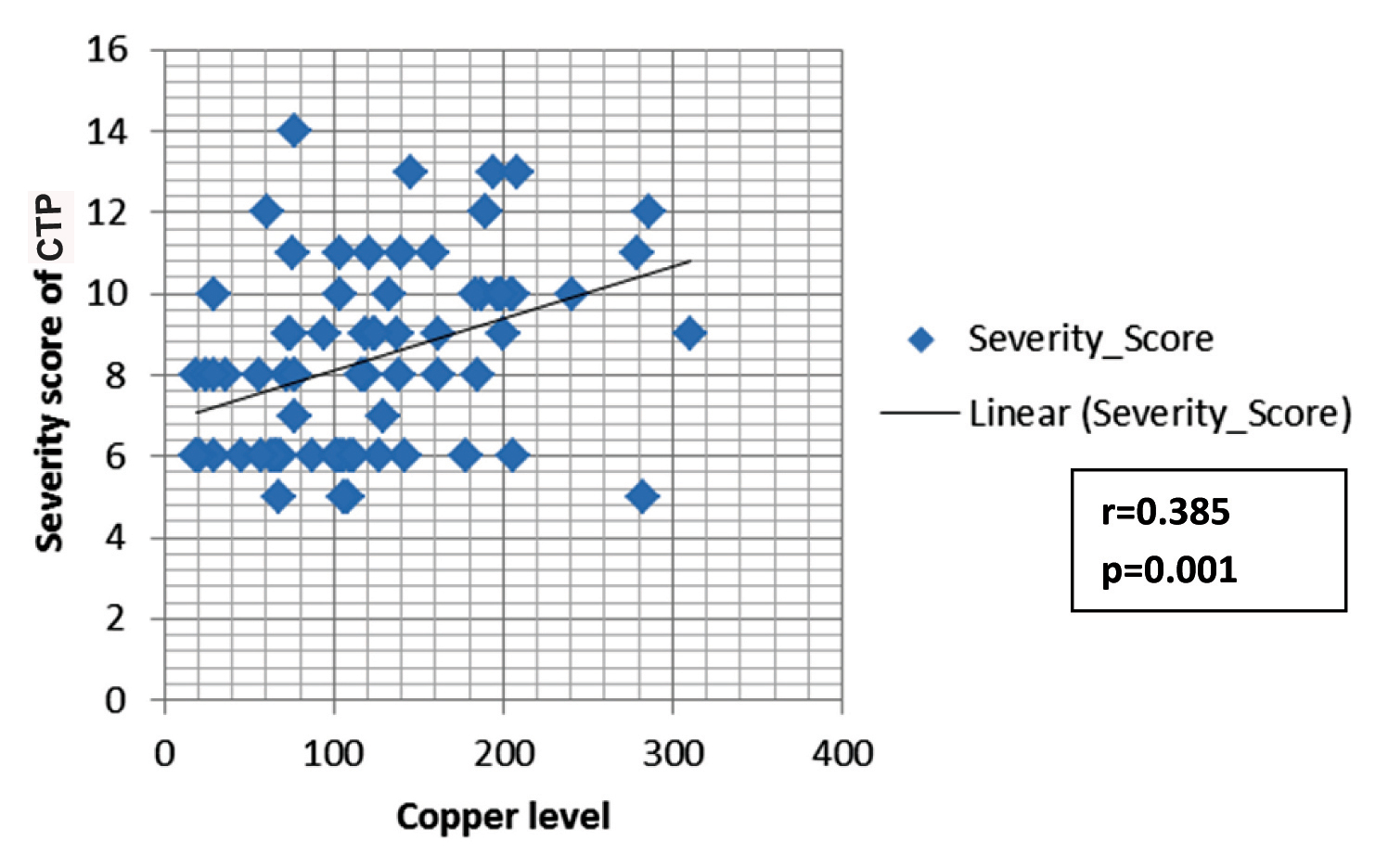
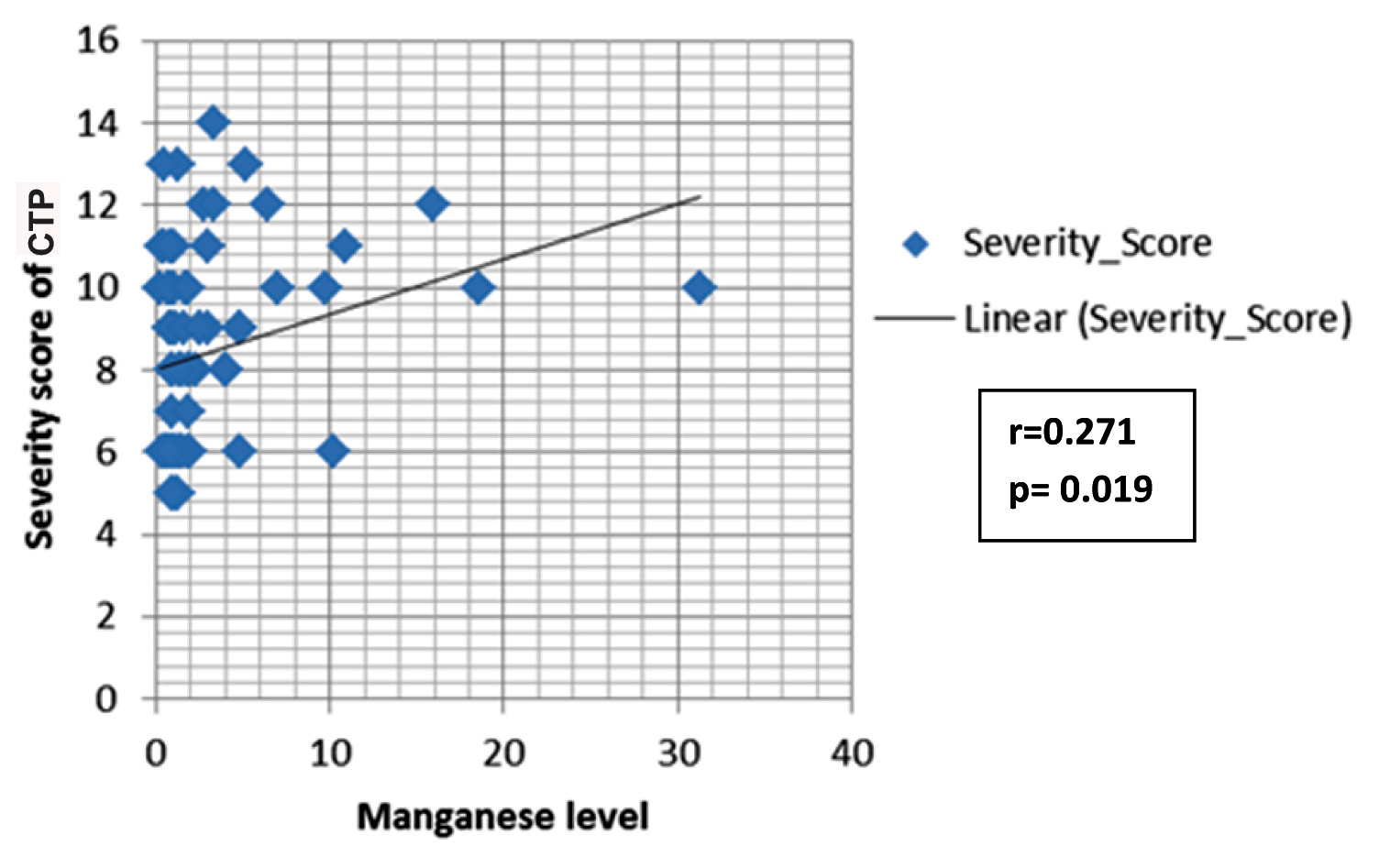
The mean age for the CTP groups were 48.6 ± 12.1 years, 54±11.7 years and 48.6±12.1 years for Class A, B, and C respectively. The comparison of CTP groups regarding trace elements and albumin is shown in [Table/Fig-1]. The concentration of zinc decreased with increase in severity of liver disease, and the mean difference between different CTP groups was statistically significant (p<0.001). There was a significant negative correlation between zinc and Child Score (r= -0.439; p<0.001) [Table/Fig-2]. The mean albumin was 3.69+0.32 gm/dl, 3.21+0.46 gm/dl, 2.89+0.50 gm/dl in the respective CTP class which was statistically significant (p<0.001). The mean albumin level in the serum of patients with CLD was correlated with serum zinc level and it showed a positive correlation with severity (r=0.232; p=0.045). Copper concentration was increased in patients with more severe clinical state of liver cirrhosis and mean level difference of copper among the Child-Pugh groups were statistically significant (p<0.001). Moreover, copper showed significant positive correlation with Child Pugh Score (r= 0.385; p= 0.001) [Table/Fig-3]. The serum level of manganese was significantly higher in patients with Child-Pugh C Class in comparison to patients with Child-Pugh A and B Class (p<0.05) but mean positive correlation with Child-Pugh Score (r=0.385; p<0.001). The serum level difference of manganese among the Child-Pugh groups was statistically not significant. However, manganese showed significant positive correlation with Child-Pugh Score (r=0.271; p= 0.019) [Table/Fig-4]. The concentrations of magnesium did not differ significantly between CTP class with the mean level difference of magnesium among the Child-Pugh groups not statistically significant. The trace elements were correlated to each other. Among the trace elements, a significant negative correlation was found between Zinc and Copper (r=−0.349; p=0.002), Copper and Magnesium (r=-.252; p=0.029) and Magnesium and Manganese (r=-.259; p=0.025), while other trace elements did not show significant correlation among themselves.
Comparison between CTP classes.
| Parameters | Chronic Liver Disease |
|---|
| Class A (n=25) | Class B (n=25) | Class C (n=25) | p-value | Post-hoc test value |
|---|
| Mean (SD) | Mean (SD) | Mean (SD) | A vs B | A vs C | B vs C |
|---|
| Zinc (μg/dl) | 70.15 (21.5) | 58.60 (22.13) | 40.85 (23.15) | <0.001*** | 0.167 | <0.001*** | 0.017 |
| Copper (μg/dl) | 95.97 (60.6) | 109.63 (64.42) | 164.59 (64.96) | <0.001*** | 0.727 | <0.001*** | 0.008 |
| Magnesium (mg/dl) | 2.16 (0.30) | 2.22 (0.24) | 2.06 (0.28) | 0.1 | 0.683 | 0.453 | 0.108 |
| Manganese (μg/dl) | 1.57 (1.99) | 2.09 (1.19) | 5.22 (7.32) | <0.01** | 0.910 | 0.013 | 0.039 |
| Albumin (gm/dl) | 3.69 (0.32) | 3.21 (0.46) | 2.89 (0.50) | <0.001*** | <0.001*** | <0.001*** | 0.031 |
Comparison was done using ANOVA (analysis of variance test) *p<0.05, significant; **p<0.01, very significant; ***p<0.001, indicates that groups are responsible for variance in the measured variable and is highly significant and the rest are not significant (p>0.05)
Spearmen correlation between zinc and CTP Score.
Spearmen correlation between copper and CTP Score.
Spearmen correlation between manganese and CTP Score.
Discussion
Trace elements play an important role in oxidative stress and redox potentials. A possible role of zinc, copper, manganese and magnesium in pathogenesis of CLD and its complications is still subject of researches.
Magnesium is an essential component of human body and other mammals whose role in liver cirrhosis and its complication is still a matter of research due to its functionality in the organism [8]. Magnesium is associated with many enzymatic reactions involving energy metabolism and protein and nucleic acid synthesis.
Zinc participates in more than 300 enzymatic systems [9] and is also a key player for various processes like wound healing, immune system, glucose control, digestion and fertility. Zinc is known to act as antioxidant, membrane stabilizer, cofactor in synthesis of DNA and as an anti-inflammatory agent [10]. Zinc is also involved in maintenance of human immunity by mobilizing some neutrophil function [11]. T-helper and suppressor cells, T-effector cells and T-natural killer cells are known to be zinc –dependent [12]. Decreased intake, decreased absorption, decreased bioavailability and increased losses (because of malabsorption) are major factors for low serum zinc level in patients with liver cirrhosis [13].
Copper is an important trace element and is associated with a number of metalloproteins. Reactive copper can participate in liver damage directly or indirectly, through Kupffer cell stimulation [14]. Copper is essential for various physiological processes such as cellular respiration, free radical defense, melanin pigment synthesis, connective tissue biosynthesis and metabolism of cellular iron. It is also a major component of catalytic centers of many redox enzymes [15].
Manganese is a structural part of arginase, which is an important enzyme in urea metabolism. Manganese acts as an activator of numerous enzymes in the Krebs cycle, particularly in the decarboxylation process [7].
In our study, we found progressive decrease in serum zinc in parallel to the severity of liver disease as assessed by Child’s-Pugh classification; these findings are in favour of study done by Somi M et al., who found that zinc level was significantly lower in Child’s Class B than Child’s Class A [16]. Also, Yoshida Y et al., found that zinc level was significantly lower in patients with decompensated liver cirrhosis than patients with compensated cirrhosis [17]. Our results also agree with Nakayama A et al., and Yasuyuki A et al., explaining that during the cell damage and inflammation, liver cells take up more zinc to synthesize nucleic acid, protein and enzymes related with zinc [18,19]. Zinc intake and absorption is known to decrease with liver damage progression, poor appetite, impaired function of intestines and stomach and high pressure of the portal vein. Hypoalbuminemia also results in less combination with zinc and it is easily lost through urine and sweat because of diffusion characteristic of blood zinc.
Nangliya V et al., and Kar K et al., also concluded similar findings of decrease in zinc levels with severity of liver disease and concluded that decrease in zinc level in cirrhosis could be due to low ingestion because of protein reluctance [20,21], increased loss in gastroenterological system due to diarrhea or intestinal malabsorption, and increased urinary losses [13]. Neurotransmitters like gamma- aminobutyric acid and norepinephrine are found to be altered by zinc deficiency including the alteration in urea metabolism which is known to predispose to hepatic encephalopathy. Experiments in animals with zinc supplementation were reported to lead to a reduction in blood ammonia. Zinc is also known to induce intestinal and hepatic metallothionein syntheses. Zinc decreases copper absorption by increasing the formation of Copper-metallothionein in intestinal epithelial cells [22]. Excess copper intake should be avoided while zinc supplementation should be encouraged in patients with chronic liver disease.
Lin CC et al., also implied that the mean zinc level in the serum of patients with hepatic cirrhosis was significantly lower than controls [23]. This finding is in agreement with the observation reported by Grungreiff K, who indicated that patients who have CLD, especially with liver cirrhosis, frequently have low levels of serum zinc and clinical features [24].
When results were compared for severity of liver disease, the serum copper and manganese levels were significantly increased with advancement of liver disease (CTP A>B>C), but mean level difference of manganese among the CTP groups was statistically not significant. Rahelic D et al., also concluded increased copper concentration in patients with severe liver disease explaining copper’s role in the redox process. Redox cycling between Cu2+ and Cu1+ can catalyze the production of toxic hydroxyl radicals [7]. It is a well known fact that redox processes and oxidative stress play an important role in the pathogenesis of CLD including cirrhosis.
Nangliya V et al., also found increased concentration of copper with severity of liver disease [20]. Kar K et al., not only found serum copper to be significantly raised in cirrhotic patients but furthermore, observed this increase to be significantly higher in the decompensated group in comparison to the compensated patients [21]. Nagasue N et al., implied that serum copper level was significantly higher in severe liver disease patients than in normal subjects [25]. The high copper level may be explained by increased uptake from the gut, diminished excretion by the liver, and tissue breakdown with consequent release of copper stores.
Serum levels of manganese were higher in patients with CTP C liver cirrhosis in comparison to those in CTP A and B cirrhosis but the mean difference were not statistically significant. Higher serum levels of manganese in Krieger D et al., research as well as in research of Layrargues GP et al., was also found in cirrhotic patients [26,27]. After all, it seems that serum levels of manganese are higher in patients with CLD than in healthy people. Manganese is secreted in bile so the concentration of manganese increases in cholestatic liver disease, which could be one of the possible explanations why manganese accumulation is common in CLD. Rahelic D et al., also concluded the same findings in his study [7]. It seems that manganese concentrations are higher in patients with severe liver disease possible due to the advanced intrahepatic and portosystemic shunting.
In our study, magnesium levels were similar in all CTP groups. The same observations were also witnessed by Rahelic D et al., who did not observe any significant changes in magnesium levels between liver cirrhosis patients and healthy population [7]. Kar K et al., also suggested that magnesium levels are not uniformly decreased in the cirrhotic patients depending on the degree of severity of symptoms [21].
Limitation
Our study had few limitations which include absence of a control group of healthy subjects. Secondly, the nutritional status of the subject was not known.
Conclusion
Trace element abnormalities may reflect the condition of liver dysfunction. Our results suggest that liver dysfunction may alter the metabolism of trace elements. Does correction of these metabolic abnormalities of trace elements delay or prevent complication of cirrhosis should be further evaluated.
Comparison was done using ANOVA (analysis of variance test) *p<0.05, significant; **p<0.01, very significant; ***p<0.001, indicates that groups are responsible for variance in the measured variable and is highly significant and the rest are not significant (p>0.05)