Advancement in surgical techniques and increasing age of the surgical population has witnessed an increased prevalence of CKD in patients presenting for various elective surgeries [1,2]. The association of CKD with adverse outcomes, including MACCE and death, in both surgical and non surgical settings is well established [3,4]. MACCE constitutes an important cause of severe perioperative morbidity and mortality after cardiac and non cardiac surgeries [5,6]. CKD integrates numerous pathophysiologic mechanisms that may contribute to postoperative morbidity and mortality. These include increased levels of inflammatory factors, hypercoagulability, elevated plasma homocysteine, excess arterial calcification, and endothelial dysfunction.
According to the guidelines of glomerular, the National Kidney Foundation for assessing kidney disease, estimated Glomerular Filtration Rate (eGFR) is a better overall index of renal function than serum creatinine level alone [7]. CKD measured by eGFR has been shown to be a reliable predictor of adverse outcomes including MACCE in the community-based population and in the perioperative setting [8,9]. Previous studies [9] conducted on patients undergoing elective non cardiac surgeries have shown CKD to be an independent risk factor for adverse cardiovascular events and death. However, their non cardiac surgical population was represented largely by vascular surgeries alone. There is a paucity of literature evaluating the relationship of eGFR with perioperative MACCE in apparently lower-risk patients undergoing intermediate-risk surgeries like thoracotomy.
The hypothesis of the study was that, eGFR could be a predictor of MACCE and prolonged hospital stay after elective, moderate-risk thoracic surgery procedure. Thus, the objective of the present study was to analyse the prognostic utility of preoperative eGFR in the occurrence of MACCE in patients undergoing thoracotomy. We also sought to determine a preoperative, clinically relevant eGFR cut-off value for identifying thoracotomy patients at higher risk for MACCE.
Materials and Methods
This retrospective study was conducted with the approval of the Institutional Ethics Committee and the need for individual patient informed consent was waived as no care interventions were to be performed.
Study Population
Between July 2012 and July 2016, 436 consecutive patients who underwent thoracotomy for lung pathologies were identified from the institute’s electronic database. This study duration of four years was chosen as the institutes electronic data base was updated at this time and well maintained with regular quality control audits being performed. After excluding those who were <40-year-old, pregnancy, surgical procedures related to a previous postoperative complication, 212 patients were enrolled. All enrolled patients had received combined general and thoracic epidural anaesthesia or general anaesthesia. Hospital stay for surgical causes was longer than 24 hours in all patients.
Demographic variables included age, gender, weight, comorbidities with risk factors for MACCE (unstable coronary syndromes, history of heart failure, arrhythmias, valvular disease, history of coronary artery disease, history of cerebrovascular disease, history of CKD, diabetes mellitus, abnormal ECG, cardiac rhythm other than sinus, hypertension). Preoperative serum creatinine (S.Cr) was measured in all the patients as a part of the routine laboratory investigations. The preoperative eGFR was then calculated from the routine preoperative serum creatinine measurements in each patient with the CKD-EPI equations as described in [Table/Fig-1].
CKD-EPI equation expressed for specific sex and serum creatinine levels.
| Gender | Serum creatinine | Equation for estimating GFR |
|---|
| Female | ≤ 0.7 mg dl–1 (≤62 μmol litre–1) | 144 × (SCr/0.7)–0.329 × 0.993Age |
| Female | >0.7 mg dl–1 (>62 μmol litre–1) | 144 × (SCr/0.7)–1.209 × 0.993Age |
| Male | ≤ 0.9 mg dl–1 (≤80μmol litre–1) | 144 × (SCr/0.7)–0.411 × 0.993Age |
| Male | >0.9 mg dl–1 (>80μmol litre–1) | 144 × (SCr/0.7)–1.209 × 0.993Age |
Patients were then classified into six groups according to the calculated eGFR (ml min -11.73 m -2): Stage 1, eGFR >90; Stage 2, eGFR=60–89.9; Stage 3a, eGFR=45–59.9; Stage 3b, eGFR=30–44.9; Stage 4, eGFR=15–29.9; Stage 5, eGFR <15, in accordance with staging criteria set forth in the global KDIGO guidelines. The main outcome studied was occurrence of a perioperative MACCE at any point intraoperatively or postoperatively till hospital discharge. MACCE [5,6] was defined as any of the following: acute myocardial infarction, angina, congestive heart failure, new cardiac arrhythmia, non fatal cardiac arrest, stroke, cardiovascular death, or cerebrovascular death occurring at any point from admission into operation theatre till hospital discharge. The length of hospital stay was also measured as a postoperative variable.
Statistical Analysis
Statistical analysis were performed using SPSS for Windows (version 17.0) Data were expressed as median and 25th–75th percentile for the continuous variables, and as number of cases and percentages for the categorical data. For all analyses, statistical significance was set at p < 0.05. We used the Mantel–Haenszel test for analysis and comparison of trends in MACCE and total length of hospital stay between the six eGFR subgroups. Logistic regression analysis with eGFR as the independent variable was used to calculate the Odds Ratio (OR) for MACCE.
Results
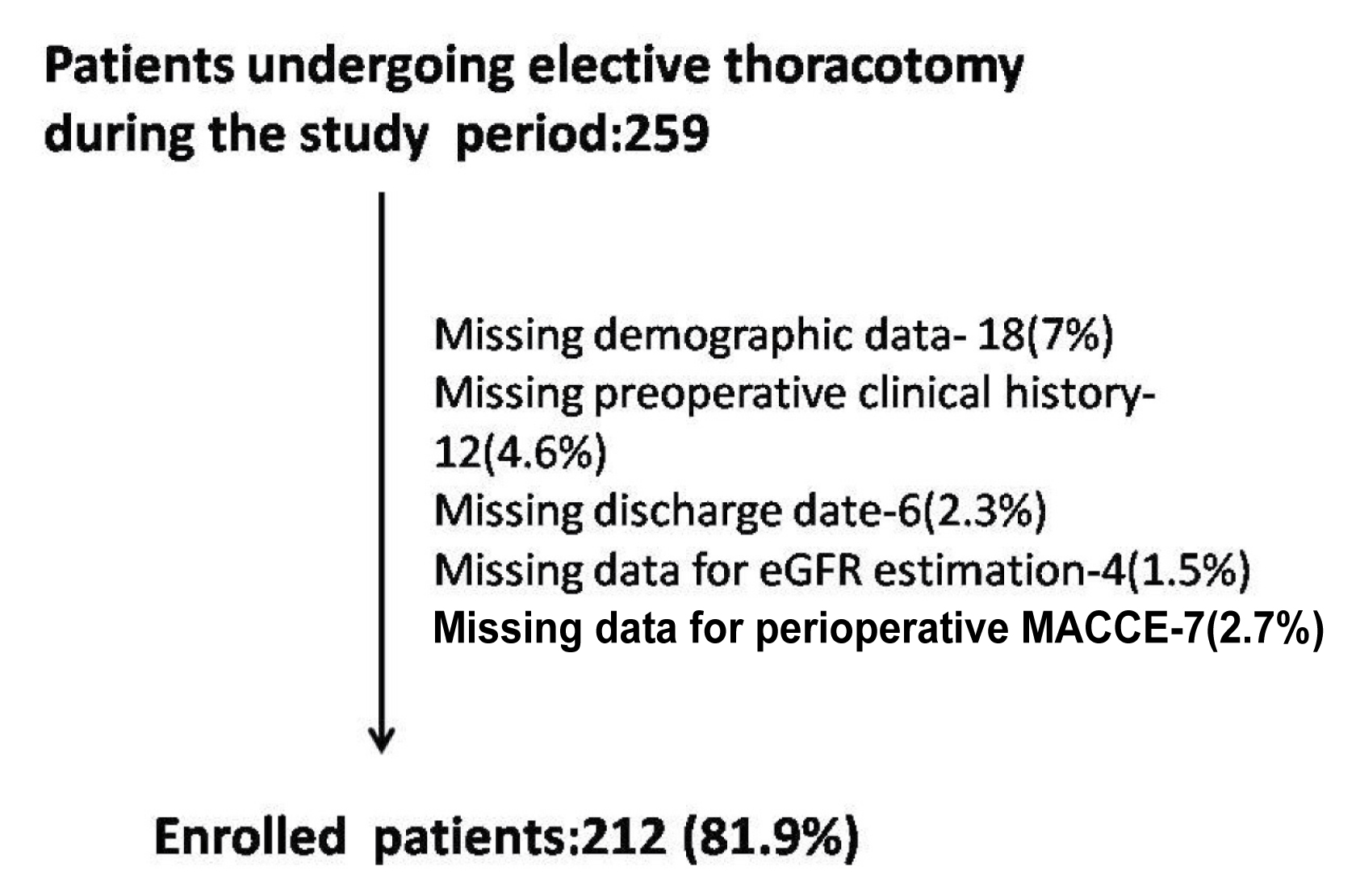
Patient profiles are given in [Table/Fig-2]. A total of 259 patients were recruited for the current study, of which 47 patients were excluded due to lack of necessary data [Table/Fig-3] and 212 patients were enrolled for the final study. When we compared baseline patient profiles across the degree of preoperative eGFR, the patients with lower categories of eGFR were older, and had higher prevalence of preoperative risk factors for MACCE. The incidence of perioperative MACCE in the current study was 4.24% (9 out of 212 patients). The mean eGFR in our study as calculated by the CKD-EPI equation was 83 ml min-11.73 m-2. The incidence of perioperative MACCE in each eGFR group is shown in [Table/Fig-4]. With the exception of nonfatal cardiac arrest which was not seen in any patient, the incidence of all MACCE increased with progressively worsening preoperative eGFR [Table/Fig-5]. It was seen by logistic regression analysis that the occurrence of any perioperative MACCE increased significantly with worsening eGFR (p<0.001). The increase in MACCE was particularly marked from stage 3b onwards to the later stages (OR 1.9 in 3a vs. 3.6 in 3b) [Table/Fig-6]. The two mortalities observed in the study were in Stage 4 and 5 with no deaths seen in the better eGFR subgroups. There was also an increased length of hospital stay with declining eGFR [Table/Fig-3].
Patient profiles and preoperative clinical risk factors according to preoperative CKD-EPI eGFR values (number of cases and %).
| Variables | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 |
|---|
| No. of patients | 93 | 77 | 33 | 6 | 2 | 1 |
| Age (years) median | 43 (40-46) | 56 (48-64) | 61 (55-67) | 70 (60-77) | 70.5 (69-72) | 77 |
| Duration of surgery(minutes)median | 270 (120-330) | 240 (180-320) | 252 (192-372) | 250 (196-300) | 280 (250-310) | 276 |
| Diabetes mellitus | 2 (2.2) | 3 (3.9) | 2 (6) | 2 (33.3) | 2 (100) | 1 (100) |
| Hypertension | 3 (3.2) | 2 (2.6) | 3 (9.1) | 3 (50) | 2 (100) | 1 (100) |
| History of ischemic heart disease | 0 (0) | 1 (1.3) | 2 (6) | 1 (16.7) | 1 (50) | 1 (100) |
| History of heart failure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| History of cerebrovascular disease | 1 (1.1) | 0 (0) | 1 (3) | 1 (16.7) | 1 (50) | 0 (0) |
| Arrythmias | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Postoperative length of stay, (days) median | 3 (2-7) | 4 (2-6) | 4 (2-8) | 7 (6-10) | 9 (8-10) | 12 (12) |
Patient recruitment flowchart.
Distribution of patients according to eGFR and perioperative MACCE.
| eGFR (ml min-1 1.73 m-2) | eGFR CKD-EPI |
|---|
| Patients | n (%) MACCE |
|---|
| 1 (>90) | 93 | 0 (0) |
| 2 (60-89.99) | 77 | 1 (1.3) |
| 3a (45-59.99) | 33 | 4(12.12) |
| 3b (30-44.99) | 6 | 2 (33.3) |
| 4 (15-29.99) | 2 | 1(50) |
| 5 (<15) | 1 | 1(100) |
Absolute number of cases (%) of perioperative MACCE. (# Mantel Haenszel test for trends).
| eGFR | 1 | 2 | 3a | 3b | 4 | 5 | p-value # |
|---|
| nAnginaAMICCFArrhythmiaNon fatal cardiac arrestAcute cerebrovascular accidentCardiac deathNon cardiac death | 9300000000 | 77001 (1.3)00000 | 332 (6.1)1 (3)02 (6.1)01 (3)00 | 62 (33.3)01 (16.7)2 (33.3)01 (16.7)00 | 201 (50)1 (50)1 (50)001(50)0 | 10001 (100)01 (100)01 | 0.0050.0530.0270.000-0.0040.0770.026 |
Logistic regression for MACCE; OR (95%) CI for any MACCE.
| eGFR stage | Any MACCE odds ratio |
|---|
| Stage 1Stage 2Stage 3aStage 3bStage 4Stage 5 | 11.7 (1.1-2.6)1.9 (1.1-3.2)3.6 (1.9-7.8)4.7 (1.8-10.9)5.9 (1.8-15) |
Discussion
In the present study, we analysed the relationship between eGFR and perioperative MACCE in elective thoracic surgeries. The important findings of this study were: 1) the incidence of perioperative MACCE including mortality increased with progressively worsening eGFR; 2) there was a graded relationship between MACCE and the stage of CKD; 3) logistic regression analysis showed that the incidence of MACCE markedly increased from Stage 3b (CKD-EPI eGFR<45 ml min -11.73 m -2) onwards; 4) the duration of postoperative hospitalization after thoracotomy increased with worsening eGFR stages. It is the anaesthesiologist’s responsibility to identify patients at elevated risk during the preoperative period and then to stratify perioperative management in the high-risk population to improve their outcome. Our study points towards the routine use of preoperative CKD-EPI eGFR for cardiovascular and cerebrovascular risk assessment in patients undergoing thoracotomy.
Most of the studies [9] showing CKD to be an independent predictor of MACCE have been performed in the cardiac and vascular surgical population. The need for further validation of the findings in moderate risk non cardiac, non vascular population was highlighted in most of these studies. Literature regarding the incidence of MACCE in elective non cardiac surgical population is limited [5,6,9] and thoracic surgeries were poorly represented in these studies. Moreover, most of these studies [5,6,9] used either serum creatinine alone or the old Cockcroft–Gault equation to estimate GFR. To our knowledge, ours is the first study to analyse the relationship between eGFR and MACCE in the thoracic surgery population using the currently KDIGO recommended CKD-EPI equation as an estimate of eGFR. Go and associates [8] reported close relationship between eGFR and MACCE in a large community-based population with a higher increase in event rates for eGFR <45 ml min -11.73 m-2. Though this study was performed in the non surgical setting, the trend of MACCE with eGFR was similar as in our study. The plausible explanation for this is that the prevalence of cardiac disease increases with worsening CKD. This is consistent with our study results, of higher prevalence of preoperative risk factors for MACCE with progressive renal impairment.
The meta-analysis by Mooney JF et al., [9] found that there was a powerful relationship between eGFR, and both short-term and long-term prognosis after, predominantly cardiac and vascular, surgery. The need for future studies in the non cardiovascular population was stressed upon. Ackland GL et al., [11], found that a preoperative eGFR < 50 ml/min/1.73m2 (calculated by the Modification of Diet in Renal Disease {MDRD} study equation) was associated with increased perioperative morbidity and hospital stay in patients undergoing elective orthopaedic surgeries. In our study also, we found an eGFR of <45 ml/min/1.73m2 to be associated with increased incidence of MACCE. Mases A et al., in a post-hoc analysis of a previous study outcome found similar results in a large population of non cardiac surgical population [6]. However, their study population included a mixture of both intermediate to high risk surgeries and thoracic surgeries were represented by a mere 3.9% of the total study group.
Ours is the only study to look at the incidence of MACCE in a targeted intermediate risk patient population using the CKD-EPI equation to calculate eGFR. Though serum creatinine has been one of the oldest surrogates for GFR, it is affected by factors such as age, gender, ethnicity, diet, muscle mass and medication. Moreover, serum creatinine doesn’t increase beyond the normal range until at least 50% of renal function is lost [12,13]. The other creatinine based formulae for GFR estimation used widely in research and clinical practice are not without disadvantages. The most important shortcoming of both Cockcroft-Gault and MDRD equations is that both formulae have lower precision in people with normal GFR. Cockcroft-Gault overestimates and MDRD underestimates the measured GFR at higher values (GFR >60 ml/min/1.73m2). Unlike the other two formulae, CKD-EPI was developed through several studies in populations with suboptimal renal function and is advantageous in its probable improved cardiovascular risk prediction compared to MDRD in a middle-age population with borderline renal dysfunction [3,14]. Monitoring for the development of postoperative acute renal failure, which might be precipitated by intraoperative haemodynamic perturbations or blood loss was beyond the scope of this study and was not one of its objectives. Both the two mortalities observed in the study were in patients in preoperative stages 4 and 5 with eGFR values <30 ml min -11.73 m-2 and had an uneventful intraoperative course. None of the patients in the study had major haemodynamic perturbations and the institutes transfusion protocols were followed in order to maintain Hb of >9 gm/dl in all patients.
The chief strength of this study is the collection of postoperative MACCE data in a non cardiac surgical population undergoing homogeneous, moderate-risk surgery in which the impact of various stages of CKD on MACCE outcomes has not undergone prior systematic evaluation.
We are the first to use the currently recommended CKD-EPI classification of CKD to explore the relationship between various stages of eGFR and MACCE in the intermediate risk thoracic population.
Limitation
This is a retrospective study from a single centre; however the objective nature of both the measurements and outcomes should reduce any tendency towards bias. Our study data can only provide associative conclusions as no interventions were assessed. There were also some restrictions in our measures of renal function. We calculated eGFR from a single value of serum creatinine. We have also not analysed albuminuria, which could further enhance the predictive value of CKD. As the superiority of CKD-EPI equation over the other formulae is more or less established, it was used alone without comparing it with other formulae. However, the present study has a limitation in not using Cystatin C [15], an index of glomerular function, which may be a better predictor of cardiovascular outcomes, particularly in elderly patients. Further studies comparing risk prediction with CKD-EPI eGFR and the Cystatin C based method is warranted. Finally, the study data was limited to in-hospital events and in-hospital mortality and did not provide insights about mid term to long term outcomes.
Conclusion
We have elucidated that preoperative eGFR is a predictor of perioperative MACCE in homogenous moderate risk elective surgical population like thoracic surgeries. There is an inverse relationship between eGFR and MACCE, particularly manifested at eGFR values <45 ml min-11.73 m-2.
Incorporation of this relatively simple tool during the pre-anaesthetic evaluation to predict perioperative MACCE, and thus provide targeted preventive strategies, even in the moderate risk, non cardiac surgical subpopulation could be beneficial.