Diffuse Large B-Cell Lymphoma (DLBCL) is the most common histological subtype of Non-Hodgkin’s Lymphoma (NHL). Primary retroperitoneal DLBCL is uncommon and has seldom been reported. Extrinsic compression of the duodenum due to lesions originating from the retroperitoneum is also rare. We present a case of a 39-year-old man who presented with inability to tolerate oral intake, abdominal pain, an upper abdominal mass and postprandial bilious vomiting caused by a large DLBCL arising from the retroperitoneum causing extrinsic compression of the duodenum. The cause of compression was initially presumed to be a neoplasm arising from the uncinate process of the pancreas or duodenum because of its proximity to the uncinate process and apparent widening of the C loop of duodenum. Repeat Computed Tomography (CT) scans were obtained because of the rapid increase in the size of the mass, normal levels of tumour markers such as Cancer Antigen (CA) 19-9, Carcinoembryonic Antigen (CEA) and no evidence of jaundice in spite of the large size of the mass. It revealed encasement of the uncinate process of pancreas with no involvement of parenchyma of the pancreas, thereby mimicking a pancreatic tumour. The neoplastic lymphoid cells were positive for Leukocyte Common Antigen (LCA), Cluster of Differentiation (CD)20, CD10, B-cell Lymphoma 2 (Bcl-2) and were negative for Creatine Kinase (CK), CD23, CD30, Anaplastic Lymphoma Kinase (ALK) and cyclin D1, D3 and D5. The Ki67 proliferative index was greater than 95%. Retroperitoneal DLBCL although rare should be considered in cases of duodenal obstruction.
Case Report
A 39-year-old man presented to the Department of Surgery, at our institution with inability to tolerate oral intake, abdominal pain, an upper abdominal mass and postprandial bilious vomiting. This difficulty had progressed over the preceding two weeks, commencing with intolerance of solids with gradual development into dysphagia to liquids and was accompanied by an approximate 10 kg weight loss. The patient had no fever or drenching night sweats. He had no previous surgery and no significant medical history, but had a history of chronic alcohol intake and tobacco usage.
Physical examination revealed an afebrile, thinly built gentleman, with normal vital signs and no generalized lymphadenopathy. Abdominal examination revealed an upper abdominal fullness which was confirmed to be a 20 cm x 15 cm mass which was minimally tender, had smooth surface and firm consistency. The mass did not move with respiration, did not fall forward in the lateral positions and had no intrinsic mobility. The swelling had a well defined inferior and lateral boundaries, extending from the epigastrium to below the umbilicus and to the right hypochondrium. The swelling almost doubled itself during the hospital stay of 10 days.
Laboratory work-up yielded a high lactate dehydrogenase value of 449 U/L, CA 19-9 value of 3 U/ml, CEA value of 2.25 ng/ml without other abnormalities. Ultrasound identified a thin walled heterogeneous hypoechoic mass lesion in the right upper quadrant of the abdomen measuring 13.1 cm x 10.6 cm x 11.1 cm.
An acute abdominal series which included Posteroanterior (PA) erect abdomen, Anteroposterior (AP) supine abdomen and PA chest radiographs, visualized no evidence of Gastrointestinal (GI) obstruction.
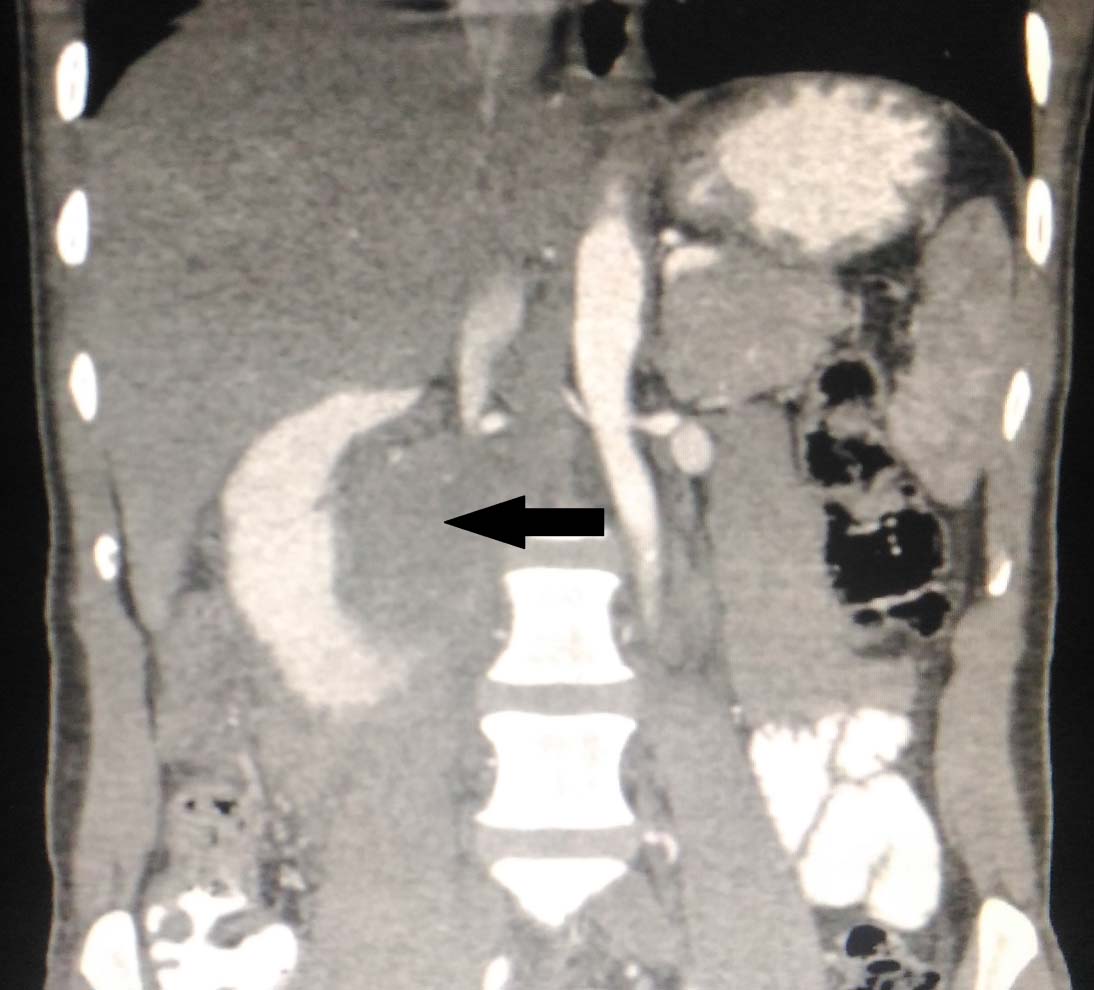
The initial Computed Tomography (CT) revealed a well-defined, lobulated, mildly enhancing soft tissue attenuating lesion in the region of the uncinate process of the pancreas causing widening of the C loop of the duodenum. The mass appeared to be extending anteriorly into the 3rd part of duodenum causing compression of bowel loops and encasement of Superior Mesentric Artery (SMA) and its branches with compression of the Inferior Vena Cava (IVC) [Table/Fig-1]. Hence, he was initially diagnosed to have an uncinate process tumour/ duodenal Gastrointestinal Stromal Tumour (GIST) based on radiographic appearance.
The neoplasm causing widening of the C loop of the duodenum. The mass appeared to be extending anteriorly into the 3rd part of duodenum causing compression of bowel loops.
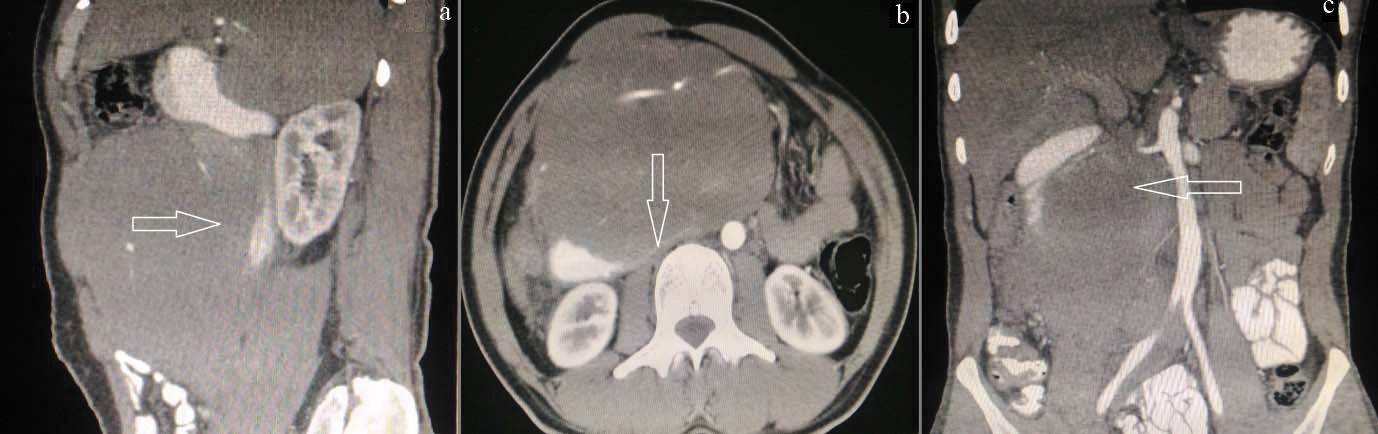
The patient underwent repeat CT scans because of the rapid increase in the size of the mass, normal levels of tumour markers such as CA 19-9, CEA and no evidence of jaundice in spite of the large size of the mass. The repeat CT revealed a homogenously enhancing rounded mass lesion measuring 14.7 cm x 14.5 cm x 9.4 cm, centered retroperitoneally, seen encasing the SMA and Superior Mesentric Vein (SMV), causing narrowing of the SMV but not occluding the vessel. The lesion was also seen to encase the uncinate process of the pancreas. There was evidence of dilatation of the 1st and 2nd part of the duodenum with posterior displacement and compression of the third part of the duodenum causing luminal narrowing. There was no involvement of the head, body or tail of the pancreas. There were no foci of calcification or haemorrhage. Multiple small perilesional lymph nodes were seen [Table/Fig-2]. While attempting an upper GI endoscopy the scope could not be passed beyond D2, however the visualized areas were normal.
a) Dilatation of the 1st and 2nd part of the duodenum with posterior displacement; b) Compression of the third part of the duodenum causing luminal narrowing; c) The neoplasm is seen encasing the superior mesenteric artery, vein and the uncinate process of the pancreas.
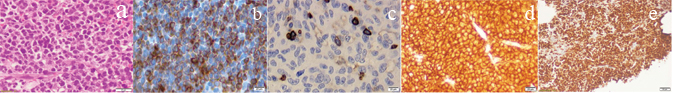
Ultrasonography guided trucut biopsies of the retroperitoneal mass were performed using 18 gauge Trucut biopsy guns. The sections taken showed fragments of a neoplasm which were small to medium in size, closely packed in sheets. The neoplastic cells exhibited high N:C ratio, with very minimal cytoplasm. The nucleus was round to oval and hyper chromatic. Single cells showed necrosis with an infiltrate of a few neutrophils with many mitotic figures. The markers done were LCA, CK, Ki67, CD5, CD3, CD20, CD23, CD 30, CD10, Bcl-2 and cyclin D1, D3, D5. The neoplastic lymphoid cells were positive for LCA, CD20, CD10, Bcl2 and were negative for CK, CD23, CD30, ALK and cyclin D1, D3 and D5. It highlights a residual small group of T lymphocytes. The Ki proliferative index was greater than 95% [Table/Fig-3].
a) The sections showing fragments of a neoplasm with small to medium cells closely packed in sheets, exhibiting high N:C ratio with very minimal hyperchromatic nuclei (H&E 40X); The neoplastic lymphoid cells were positive for Bcl2; b) LCA; c) CD20; d) (IHC 40X) and Ki Proliferative index was greater than 95%; e) (IHC 4X).
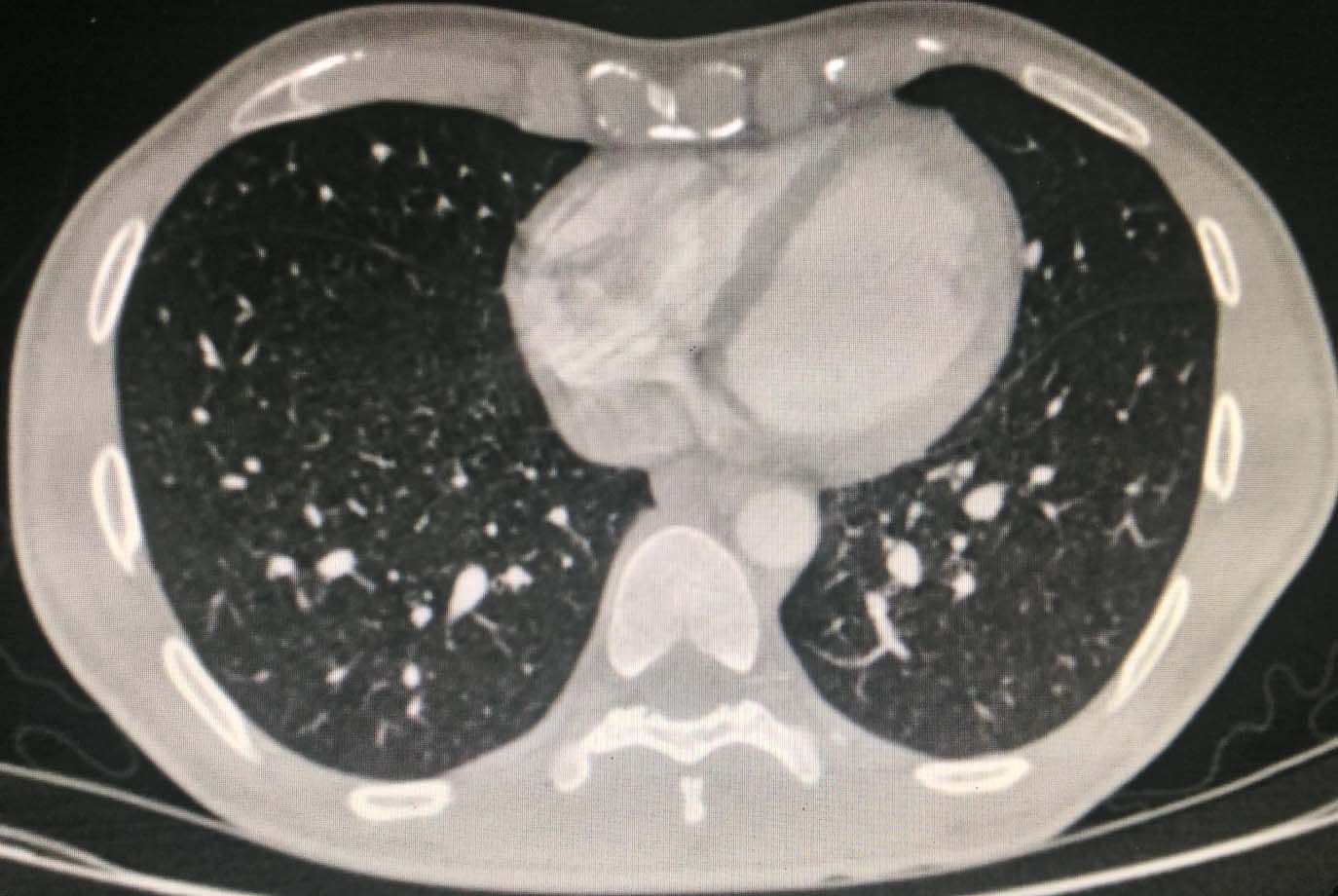
A CT scan of the brain and chest, ultrasonography of the neck and axilla and bone marrow biopsies were performed as part of the staging workup along with blood investigations such as complete blood counts with peripheral smear, renal function and liver function tests, Lactate Dehydrogenase (LDH), CEA, CA 19-9, Human Immuno Deficiency (HIV), Hepatitis B surface Antigen (HBsAG) and Hepatitis C Virus (HCV). The CT scan of the brain was unremarkable and CT chest revealed no mediastinal adenopathy, pleural or pericardial effusions and the bone marrow biopsies did not reveal any involvement [Table/Fig-4].
CT chest showing no mediastinal adenopathy, pleural or pericardial effusions.
On the basis of the Revised International Prognostic Index (R-IPI) for DLBCL, which takes into account patient age, performance status, lactate dehydrogenase, involvement of extra nodal sites, and stage, the patient’s prognosis was determined to be good (score = 2), with a predicted four year progression free survival rate of 80% and an overall survival rate of 79% [1]. The patient was started on rituximab + cyclophosphamide, doxorubicin, vincristine and prednisone (R –CHOP) regime and improved symptomatically. He had no further episodes of vomiting. There was no obvious swelling noticed in the epigastric region. The patient however relocated to a different region after the third cycle of chemotherapy, disrupting the completion of his treatment regimen at our institution. Although attempts were made to locate the patient, he was ultimately lost to follow up.
Discussion
DLBCL is the most common histological subtype of NHL [2]. Pileri SA et al., published the largest case series on primary retroperitoneal DLBCL to date consisting of nine cases [3]. Retroperitoneal DLBCL presenting as duodenal obstruction is very rare. Duodenal obstruction may be intrinsic or extrinsic. Extrinsic obstruction is usually due to compression of an otherwise normal duodenum. The obstruction may be partial or complete. The most common symptoms are abdominal pain, nausea, and postprandial bilious vomiting. The underlying pathology usually determines the pattern and rate of the onset of symptoms. Willkie’s syndrome, idiopathic retroperitoneal fibrosis, adenocarcinoma of the duodenum and duodenal duplication cysts have been described to cause isolated D3 compression. Other causes include intestinal malrotation with Ladd’s band, or volvulus, gastroduodenal duplication cysts, pseudo cysts of the pancreas, abdominal aortic aneurysm, retroperitoneal haematoma and various bezoars which constitute the benign aetiology [4-7]. In this case, the imaging was suggestive of a mass effect caused by the tumour on the third part of the duodenum causing the obstruction. Primary DLBCL arising from the retroperitoneum causing isolated D3 compression is very rare.
In our patient, a large retroperitoneal tumour led to extrinsic compression of the third part of the duodenum. There was history of recurrent vomiting 15 to 30 minutes following meals, significant weight loss and upper abdominal pain. The cause of the D3 compression was initially presumed to be a duodenal GIST arising from the uncinate process of the pancreas based on the initial CT images or malignancy because of its proximity to the uncinate process and apparent widening of the C loop of duodenum. The patient underwent repeat CT scans because of the rapid increase in the size of the mass, normal levels of tumour markers such as CA 19-9, CEA and no evidence of jaundice in spite of the large size of the mass. The repeat scans revealed encasement of the uncinate process of the pancreas by the tumour with no involvement of the parenchyma of the pancreas, thereby mimicking a pancreatic tumour.
According to the International Agency for Research on Cancer (IARC), the age standardized incidence rate of NHL among both sexes worldwide is estimated at 5.0 per 100,000 people.
The differential incidence between more and less developed regions of the world is more pronounced than that of mortality rate (2.3 and 2.7 per 100,000 respectively) [8]. In India, the predominant subtype of all NHL cases is DLBCL which constitutes 59.3%. For DLBCL, the median age at presentation was 47 years and the sex ratio was 2.7:1 (male:female). 57% of patients had generalized lymphadenopathy and 56.7% had B symptoms. Patients with Stage IV Ann Arbor disease constituted a total of 49.3%. The median overall survival was 47 months (95% CI: 3-100 months) and the median event free survival was 32 months (95% CI: 0-92 months) [9,10].
Conclusion
Primary retroperitoneal DLBCL is a rare entity Gastrointestinal obstruction triggered by primary retroperitoneal, DLBCL is uncommon and has rarely been mentioned in medical literature. Early diagnosis and commencement of chemotherapy is vital to ensure good prognosis, especially in aggressive tumours with limited systemic involvement such as in our case.
[1]. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOPBlood 2007 [cited 2017 May 8] 109(5):1857-62.Available from: http://www.ncbi.nlm.nih.gov/pubmed/17105812 [Google Scholar]
[2]. Stewart BW, Wild C, International Agency for Research on Cancer. World Health OrganizationWorld cancer report 2014. International Agency for Research on Cancer 2014 [cited 2017 May 8] :916Available from: https://shop.iarc.fr/products/wcr2014 [Google Scholar]
[3]. Pileri SA, Zinzani PL, Ascani S, Orcioni GF, Gamberi B, Piccioli M, Diffuse large B-cell lymphoma with primary retroperitoneal presentation: clinico-pathologic study of nine casesAnn Oncol Off J Eur Soc Med Oncol 2001[cited 2017 May 8] 12(10):1445-53.Available from: http://www.ncbi.nlm.nih.gov/pubmed/11762818 [Google Scholar]
[4]. Felton BM, White JM, Racine MA, An uncommon case of abdominal pain: superior mesenteric artery syndromeWest J Emerg Med 2012 [cited 2017 May 8] 13(6):501-02.Available from: http://www.ncbi.nlm.nih.gov/pubmed/23358897 [Google Scholar]
[5]. Shaheen O, Sara S, Safadi MF, Alsaid B, Duplication cyst in the third part of the duodenum presenting with gastric outlet obstruction and severe weight lossCase Rep Surg 2015 [cited 2017 May 8] 2015:01-04.Available from: http://www.hindawi.com/journals/cris/2015/749085 [Google Scholar]
[6]. Kermani TA, Crowson CS, Achenbach SJ, Luthra HS, Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomesMayo Clin Proc 2011[cited 2017 May 8] 86(4):297-303.Available from: http://www.ncbi.nlm.nih.gov/pubmed/21454732 [Google Scholar]
[7]. Tavakkoli A, Ashley SW, Zinner MJ, Small Intestine. In : Schwartz SI, Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, et alSchwartz’s principles of surgery 2014 10th EditionNew YorkMcGraw Hill:1146-1147. [Google Scholar]
[8]. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J cancer [Internet 2015[cited 2017 May 8] 136(5):E359-86.Available from: http://doi.wiley.com/10.1002/ijc.29210 [Google Scholar]
[9]. Nimmagadda RB V, Digumarti R, Nair R, Bhurani D, Raina V, Aggarwal S, Histopathological pattern of lymphomas and clinical presentation and outcomes of diffuse large B cell lymphoma: A multicenter registry based study from IndiaIndian J Med Paediatr Oncol 2013[cited 2017 May 8] 34(4):299-304.Available from: http://www.ncbi.nlm.nih.gov/pubmed/24604961 [Google Scholar]
[10]. Khera R, Jain S, Kumar L, Thulkar S, Vijayraghwan M, Dawar R, Diffuse large B-cell lymphoma: experience from a tertiary care center in north IndiaMed Oncol 2010[cited 2017 Jun 30] 27(2):310-18.Available from: http://link.springer.com/10.1007/s12032-009-9211-12 [Google Scholar]