COPD is characterized by irreversible obstruction of the airways with progressive reduction in airflow secondary to an abnormal inflammatory response of the lungs to inhalation of noxious particles or toxic gases [1]. COPD is a common preventable and treatable disease affecting millions of people worldwide; and COPD exacerbations and related mortality pose a major socio economic burden to the community and the nation as a whole. As per WHO estimate, nearly 65 millions of people are suffering from COPD; this contributes to 5% of all death globally [2]. By 2030, COPD will occupy fifth rank in terms of burden of disease and third in terms of mortality [3]. Data regarding prevalence, morbidity and mortality of COPD from developed countries are enormous. On the contrary, limited data is available from low and middle income countries where nearly 90% of COPD related deaths do take place among adult population (≥ 40 years) [4]. Approximately, 30 million people are suffering from COPD in India; and COPD related mortality is nearly four times than that of Western population. Mortality due to COPD is rising faster; and more even compared to that due to infectious diseases [5,6].
Inflammation and inflammatory cytokines play a major role in the pathogenesis of COPD and its exacerbation [7]. Localized airway inflammation recruits inflammatory cells such as neutrophils, macrophage, and cytotoxic T lymphocytes; exacerbates oxidative stress by production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS); and enhances apoptosis which altogether contribute to further exacerbation. Various inflammatory cytokines such as IL-1, IL-3, IL-6, TNF–α, TGF–β are elevated in the disease process. TNF-α, IL-1β, and IL-6 amplify the inflammatory process and contributed to the some of systemic effects of COPD [8,9]. TGF-β induces fibrosis of small airways. Chronic airway inflammation leads to parenchymal destruction, decreased elastic recoil of lung alveoli producing airflow limitation. Localized airway and respiratory inflammation gradually progress to systemic inflammatory response. Studies have shown that increase in cytokine concentration is a reliable predictor of near future exacerbation [10].
Serum UA, a product of purine catabolism, is a major extracellular antioxidant present in the respiratory tract. As respiratory tract is exposed to higher level of oxidative stress due to cigarette smoking, biomass fuel, industrial pollution; antioxidant like UA, ascorbic acid, α-tocopherol, ferritin lining the epithelium provides important defense against these oxidants [11]. Hyperuricaemia has been implicated in the pathogenesis of several human diseases with systemic inflammation; notably gout, vascular diseases such as atherosclerosis, hypertension, metabolic syndrome, as well as in various malignancies such as cancer of lungs, hematological malignancies, etc., [12]. Hyperuricaemia has also been implicated in various respiratory diseases like pulmonary arterial hypertension and obstructive sleep apnea [13]. But studies on the role of UA in the pathogenesis of COPD are limited; and these all have been found to be inconclusive. Besides these, the cause–effect relationship between hyperuricaemia and COPD seems to be complexed and is poorly understood. Hyperuricaemia has been reported to be both protective as well as detrimental in patients with COPD and its exacerbations [13-20]; thus warranting further prospective studies. In this manuscript, we aim to study the association of uric acid in patients with COPD in a cohort of Southern Indian population with brief review of relevant literature.
Materials and Methods
After obtaining the approval from the Institutional Ethical Committee (IEC:RC/16/24), a hospital based case–control study was conducted in the Department of Biochemistry and Pulmonary Medicine at Pondicherry Institute of Medical Sciences, Puducherry, India, over a period of two months (1st June 2016 to 31st July 2016). Written and informed consents were obtained from the participants prior to this study; and patient confidentiality was strictly maintained.
The study included 39 diagnosed stable COPD cases (age group; 40 to 60 years) based upon spirometric studies. A clinical diagnosis of COPD was made in all cases who presented with persistent cough with or without sputum production and breathlessness. This was followed by spirometric evaluation; and post bronchodilator Forced Expiratory Volume (FEV1) to Forced Vital Capacity (FVC) ratio (FEV1/FVC) less than 0.7 confirmed the diagnosis of persistent airflow limitation and thus COPD [1]. Forty six age and gender matched, nonsmoker, nonalcoholic subjects who came for routine health checkup; and had no history of any respiratory signs or symptoms (in the last three months) were enrolled as control subjects. Subjects of COPD having acute exacerbation and those with other co-morbidities which are likely to influence uric acid level such as chronic kidney disease, diabetes mellitus, decompensated liver disease, malignancy, and gout were excluded from the present study.
GOLD criteria based upon spirometry, airflow limitation were used for categorizing COPD cases as mild, moderate, severe and very severe grades (GOLD 1-mild, GOLD 2-moderate, GOLD 3-severe, GOLD 4-very severe). Post bronchodilator ratio of forced expiratory volume (FEV1) to Forced Vital Capacity (FVC) less than 0.7 was used to determine airflow limitation [1]. Grading of severity as per GOLD criteria is as follows: GOLD 1: mild (FEV1 ≥ 80% predicted), GOLD 2: moderate (50% ≤ FEV1 < 80% predicted), GOLD 3: severe (30% ≤ FEV1 < 50% predicted); and GOLD 4: very severe (FEV1 < 30% predicted) [1]. Detailed epidemiological information along with history regarding age, gender, history of smoking, alcohol intake, exposure to biomass fuel, occupational exposure (such as farmers, mine workers), duration and severity of disease, were collected from medical records as well as by direct communication with the participants. Following an overnight fast, 2 ml of venous blood was collected in plain (red topped) vacutainer from anterior cubital vein under aseptic condition in all subjects. Following clot retraction, serum was separated by centrifugation at 3000 rpm for 10 minutes. Serum UA was then measured by enzymatic colorimetric assay at 552 nm in fully automated clinical chemistry analyser (Cobas Integra 400+, Roche, Germany) using commercially available kits from Roche Diagnostics as per manufacturer’s protocol [21].
Statistical Analysis
The data was analysed by SPSS software version 20.0. Quantitative data was presented as mean ± standard deviation (SD). Student t-test was used to evaluate the difference in UA level between cases and controls; and between male and female COPD cases. One way ANOVA was used to compare UA level in different groups based on duration and stage of COPD. Kruskal –Wallis test was used to see the difference in UA level according to GOLD criteria stages of COPD. Post-hoc multiple comparisons was done by Least Significance Difference (LSD) method. A p-value less than 0.05 was considered as statistically significant.
Results
The present study included 39 cases {males; 21(53.8%), females; 18(46.2%)} and 46 controls {(males; 26(56.5%), females; 20(43.4%)}. The mean age of cases and controls was 62.97±11.30 vs. 48.76±12.71 years, respectively (p<0.001). COPD cases had significantly higher level of UA compared to control subjects (4.85±1.67 vs. 2.32±0.93 mg/dl, respectively, p<0.001) [Table/Fig-1]. Female subjects with COPD had higher levels of UA compared to their male counterparts (5.15±1.89 vs. 4.59±1.45 mg/dl, respectively) and this difference was statistically insignificant (p=0.3) . COPD cases with duration of the disease > 10 years were having higher UA level compared to those with <5 years and 5-10 years of the disease, which was statistically significant (p<0.03) [Table/Fig-2,3]. Mean UA level was compared with different stages of COPD according to GOLD criteria. Stage 4 COPD subjects had higher UA levels compared to other stages. However, the difference in UA level between different stages was not statistically significant (p=0.286) [Table/Fig-4,5]. Out of 39 cases of COPD, 13 cases were smokers and 26 cases were non smokers. In this study, we observed that non-smokers were having higher uric acid level than smokers (5.13±1.81 vs. 4.27±1.20 mg/dl). But the difference was not statistically significant. Alcohol intake did not show any alteration in uric acid level {4.9± 1.8 (alcoholic) vs 4.8±1.5 mg/dl (nonalcoholic), p=0.792}. Of the 39 cases, 12 had exposure to biomass fuel (mean serum UA: 4.6 ± 0.88 mg/dl) which was similar among those who were not exposed to the same (n=27, mean serum UA: 4.9±1.93 mg/dl) (p=0.595).
Comparison of age and serum uric acid level between COPD cases and control.
| Parameters | Control (N=46) | COPD cases (N=39) | p#-value |
|---|
| Gender-Males/females | 26/20 | 21/18 | 0.3 |
| Age in years (mean ± SD) | 48.76 ± 12.71 | 62.97 ± 11.30 | < 0.001 |
| Serum uric acid (mg/dl) (mean ± SD) | 2.32 ±0.93 | 4.85± 1.67 | < 0.001 |
COPD; chronic obstructive pulmonary disease, N; number of cases and control, SD; standard deviation, #; Student t test.
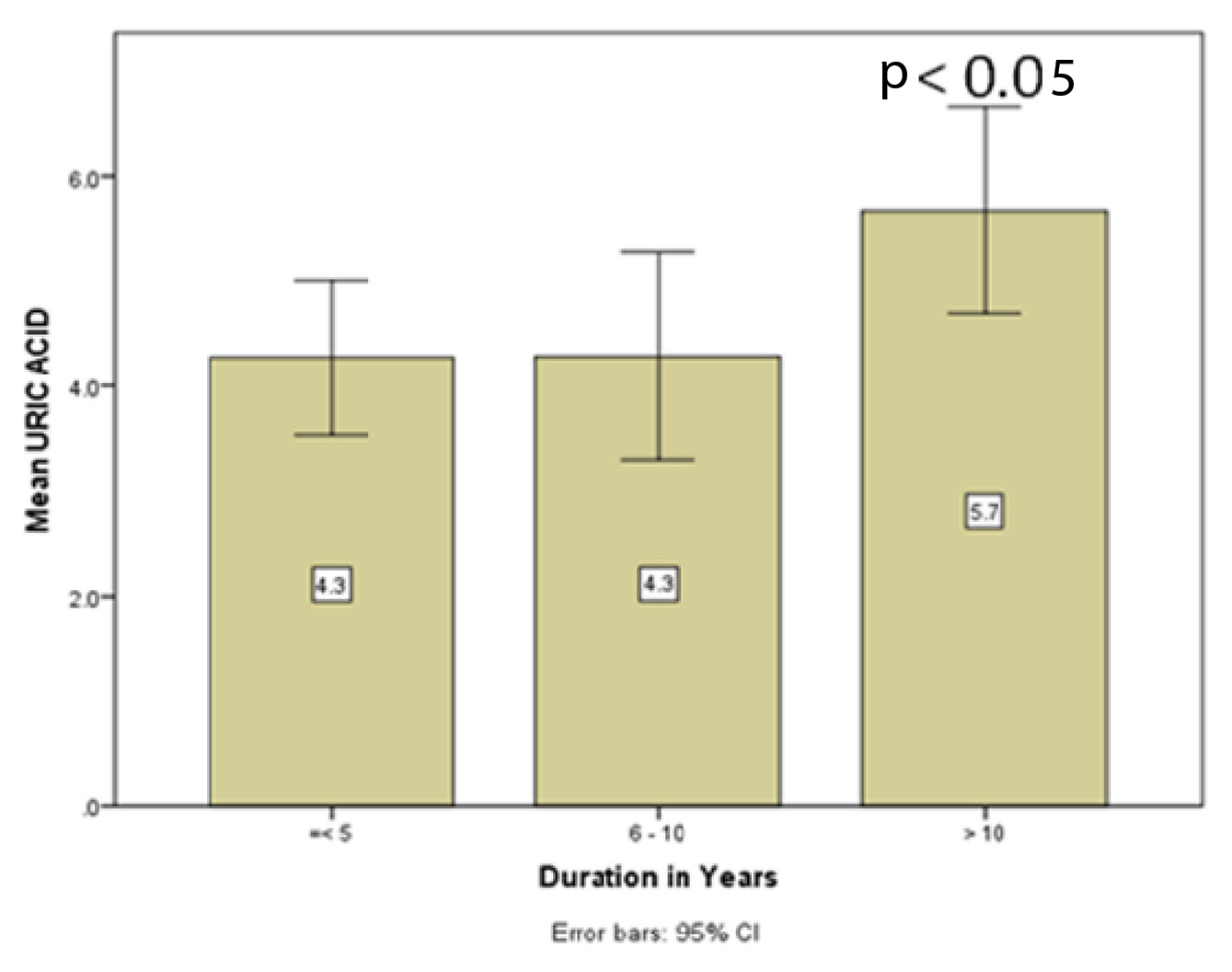
Correlation of serum uric acid level with duration of COPD.
| Duration of COPD | N | Serum UA (mg/dl) (mean± SD) | SE | 95% CI | Minimum-Maximum | p-value |
|---|
| < 5 years | 12 | 4.27 ±1.15 | 0.33 | 3.53-5.01 | 2.4-6.3 | 0.03 |
| 5-10 years | 11 | 4.28 ±1.47 | 0.44 | 3.28-5.27 | 2.2-7.4 |
| >10 years | 16 | 5.67 ±1.85 | 0.46 | 4.68-6.62 | 2.9-10.1 |
| Total | 39 | 4.85 ±1.67 | 0.26 | 4.30-5.39 | 2.2-10.1 |
COPD; chronic obstructive pulmonary disease, N; number of cases, UA; uric acid, SD; standard deviation, SE; standard error, CI; confidence interval.
Bar diagram showing uric acid level with increased duration of the disease.
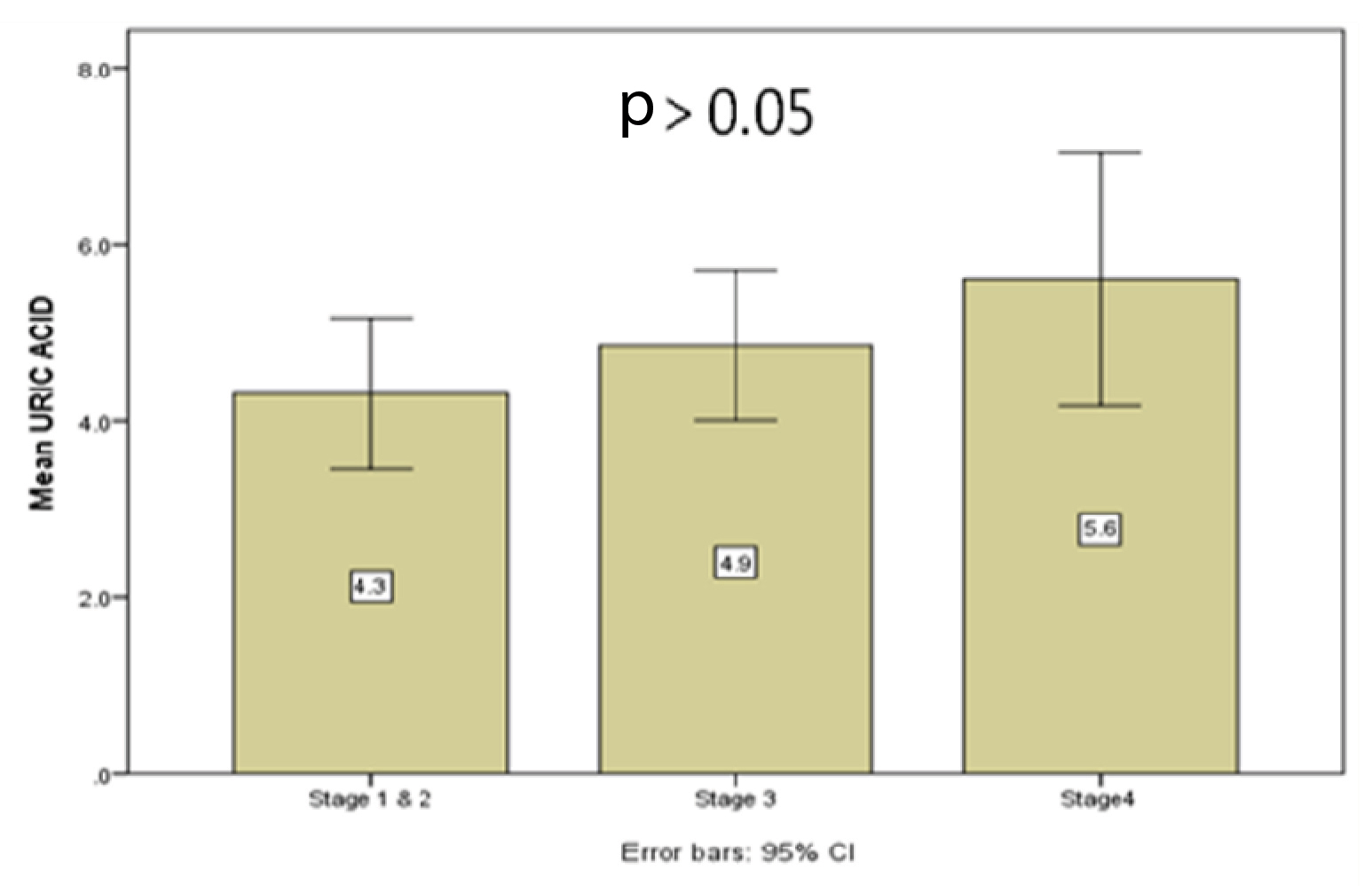
Comparison of serum uric acid level with different stages of COPD as per GOLD criteria [1].
| Stages of COPD | N | UA level (mg/dl) (mean ± SD) | p-value |
|---|
| Stages 1and 2 | 11 | 4.30 ±1.27 | p = 0.286 |
| Stage 3 | 20 | 4.85 ±1.80 |
| Stage 4 | 8 | 5.6 ± 1.71 |
| Total | 39 | 4.85 ± 1.67 |
COPD; chronic obstructive pulmonary disease, GOLD; Global Initiative for Obstructive Lung Disease, N; number of cases, UA; uric acid, SD; standard deviation.
Comparison of uric acid level in between cases belonging to different stages of COPD as per GOLD criteria (stage 1 and 2, stage 3, stage 4).
Discussion
Due to lack of uricase enzyme in humans, uric acid cannot be converted to urea which in turn, leads to nearly 50 times increased level of UA in comparison to non primate mammals. It is the most abundant antioxidant present in plasma; and at an average concentration of 5 mg/dl, this exhibits powerful antioxidant properties [15]. As an antioxidant; UA plays an important role in protecting airways from oxidative stress by inhibiting lipid peroxidation and scavenging reactive oxygen and nitrogen species [7-10].
In the present study, we noted that non smokers outnumbered the smokers; and paradoxically non-smokers had higher (p>0.05) uric acid level than smokers. In one study, Lamprecht B et al., reported that never smokers accounted for 1/4th to 1/3th of all COPD cases [22]. Among non smokers, females outnumbered the males which could possibly explained by more exposure to indoor air pollution, occupational exposure to organic dust in work place, and passive smoking; all together contributing to recruitment of inflammatory cells and further progression of COPD from moderate to severe degree [22].
Several previous studies have reported a complex interaction between hyperuricaemia and COPD and are briefly presented in [Table/Fig-6] [13-18,20,23-25]. An imbalance between oxidant/antioxidant status favors oxidative stress induced injury of the airways. Proinflammatory effect of UA with increased serum concentration has also been proposed as a cofactor in pathogenesis of COPD [12]. Pro-oxidant effects of uric acid with raised level in free-radical generation, inflammation predominates over its antioxidant effects [17]. In accordance with previous studies the present study showed significantly increased serum UA levels among COPD cases compared to controls (p<0.05) [16,17]. In contrast to studies by Aida Y et al., we observed a statistically insignificant increase in serum UA levels among female COPD patients compared to males [17].
Association of serum uric acid in subjects with Chronic Obstructive Pulmonary Disease (COPD): a brief literature review.
| Author, Reference | Place, year | Study subjects | Outcomes | Remarks |
|---|
| Bartziokas K et al., [13] | Greece, 2014 | 314; COPD(all current and ex-smokers)170; frequent exacerbators144;nonfrequent exacerbators148; UA ≥ 6.9mg/dl166; UA ≤ 6.9mg/dl | Increased UA led to increased airflow limitation, exacerbation, prolonged hospital stay, noninvasive ventilation, and ICU admission.UA increased in frequent exacerbators (N=170) than nonfrequent exacerbators (N=144) (p<0.001). | GOLD 1 and 2; lower UAGOLD 3 and 4; higher UAUA ≥ 6.9 mg/dl is an independent predictor of 30 days mortality in multivariate regression analysis. |
| Embarak S et al., [14] | Egypt, 2014 | 115; AECOPD | High UA levels on admission (≥ 6.9 mg/dl) were associated with increased 30 day mortality, prolonged hospitalization, and increased airflow limitation. | |
| Horsfall LJ et al., [15] | UK, 2014 | 3901; COPD1015; lung cancer (smoker and nonsmoker),Age group; 40 to100 years(males and females) | 100 μmol/L increase in uric acid level was associated with nearly 2% decrease in incidence of COPD and a 5% decrease in lung cancer (p>0.05) | Current heavy smokers with lower UA quintile have increased risk of lung cancer and COPD. |
| Zhang X et al., [16] | China, 2015 | 102; COPD(34; with increased UA68; with normal UA)(age and gender matched) | COPD with increased UA level had increased risk of mortality in comparison to COPD with normal UA (p= 0.005). | Hyperuricaemia is a predictor of early mortality in COPD. |
| Aida Y et al., [17] | Japan, 2011 | 2917 subjects (male and female), annual health checkup community based. | UA level increased significantly in males than females (p < 0.001)FVC and FEV (predicted) related inversely with UA in females (p<0.05) than in males (p>0.05). | Decreased lung function; predictor of increased UA in general population. |
| Garcia-Pachon E et al., [18] | Spain, 2007 | 59; stable COPD without co-morbidity. | Increased UA to creatinine ratio associated with decreased FVC (p = 0.028), FEV1 (p = 0.019); and increased dyspnoea (p=0.011). | UA to creatinine ratio is a predictor of outcome in COPD subjects. |
| Nicks ME et al., [20] | USA, 2011 | 367; smokers with COPD136;smokers with normal lung function | Decreased UA associated with increased severity of COPD (p<0.002).Decreased reduced glutathione (GSH) linked to increased COPD exacerbation (p=0.03).Plasma ascorbate; not associated with COPD. | Hyperuricaemia; linked to better lung function.Decreased GSH- leads to exacerbation of COPD. |
| Rahman I et al., [23](Multicentric study) | UK, 2000 | 95; stable COPD82; healthy smokers37; healthy nonsmoker (control) | Antioxidant capacity of UA decreased in COPD subjects and healthy smokers. | p<0.001 |
| Sato N et al., [24] | Japan, 2003 | 91; COPD(24; died of AECOPD)(mean follow up period; 31 months) | UA to creatinine ratio increased in non survivors.Negative correlation with nadir of oxyhaemoglobin saturation(r = -0.32, p<0.01). | UA to creatinine ratio independently and directly related to mortality in multivariate analysis (p<0.05). |
| Bhatia A et al., [25] | LucknowIndia 2016 | 98;AE-COPD(34;infrequent exacerbators64;frequent exacerbators83.5% smoker | Significant increase in UA levelsAmong frequent exacerbators than infrequent exacerbators.Significant positive association between UA with frequency of exacerbations. | Air pollution, GERD, anxiety/depression and increase IgE levels associated with AE-COPD. |
| Present study | Punducherry, India, 2016 | 39; stable COPD(13/39; smokers)46; healthy controls | Serum UA among COPD group significantly higher than controls.UA higher among female COPD cases.Hyperuricaemia is associated with increased duration (>10 years) and advanced stage (GOLD 3/4) COPD. | Hyperuricaemia may be associated with disease progression. |
Abbreviations: UK; United Kingdom, COPD; chronic obstructive pulmonary disease, UA; uric acid, AECOPD; acute exacerbation of COPD, FVC; forced expiratory volume, FEV1; forced expiratory volume, GOLD; Global Initiative for Obstructive Lung Disease, N; number of cases, ICU; intensive care unit, GERD; gastroesophageal reflux disease.
A recent Indian prospective study from Lucknow recruited 98 subjects with exacerbated COPD (81.6% males, 79.5% smokers, frequent exacerbators; 64/98, infrequent exacerbators; 34/98) [25]. Serum UA levels were significantly higher among frequent exacerbators (5.34±1.48 vs. 4.43±1.16 mg/dl respectively, p= 0.032). There was statistically significant positive correlation between serum UA and frequency of exacerbation (r=0.41937, p< 0.003). Air pollution, depression/anxiety, Gastro Esophageal Reflux Disease (GERD) and increased serum IgE levels were associated with increased exacerbations [25]. On the contrary, hyperuricaemia was recently shown to have a beneficial effect on the pulmonary functions in healthy middle aged Korean subjects [26].
Similar to previous studies, our observation also showed an increase uric acid levels with increasing severity of the disease; though the difference was not statistically significant [13]. Advanced GOLD stages (stages 3 and 4) COPD cases had higher uric acid level in comparison to stage 1 and 2. Studies have shown that significant correlation exists between hypoxemia and serum uric acid level [13,17]. Hypoxemia secondary to increased severity/ staging of the disease leads to excess accumulation of uric acid as a result of tissue destruction; which in turn further exacerbates localized airway inflammation, cytokine production, ROS generation and further progression of COPD. Hence, a vicious cycle of hyperuricaemia, COPD exacerbation, hypoxia induced increased uric acid level undermines the imbalance between antioxidant and prooxidant properties of uric acid [17]. Bartziokas K et al., in their study found that uric acid ≥ 6.9 mg/dl was an independent predictor of 30 days mortality [13]. Higher serum uric acid led to more prolonged hospitalization, increased need for non-invasive ventilation, and increased risk of acute exacerbation [13]. In another Egyptian study, Elshafey M et al., found out that increased uric acid level (≥ 8.4 mg/dl) had a higher sensitivity (89%) and specificity (80%) in predicting the mortality in patients with acute respiratory distress syndrome [19]. Another study by Embarak S et al., also validated the same among a large series of patients with acute exacerbation of COPD [14].
A recent population based cohort study from UK studied the association between serum UA and risk of COPD [15]. There were 3901 COPD cases enrolled in that study (age group; 30 to 100 years). After adjustment for age, gender, air pollution, smoking and alcohol intake, height, weight and blood pressure, each 100 μmol/L increase in uric acid level was associated with nearly 2% decrease in incidence of COPD (p>0.05). However, interaction between serum UA and smoking status was statistically significant (p<0.001). After multivariate regression analysis, the predicted incidence rate of COPD was 40% (248 vs. 140 per 10,000 person years) higher, in the lowest uric acid quintile compared with the highest quintile in current heavy smokers (≥20 cigarettes per day) after adjusting for potential confounders [15]. This study clearly showed an inverse relationship between smoking status and serum UA levels; and a higher incidence of COPD was linked to lower UA levels among heavy smokers. Analysis related to occupational exposure such as farming and mining could not be done due to low sample size.
Interestingly, our study also showed lower UA levels among smokers than nonsmokers; though the difference was statistically insignificant. This finding possibly suggests that smoking reduces the antioxidant property of UA in the upper airways leading to increase risk and/or severity of COPD. Furthermore, our study found that subjects with milder COPD (stages 1 and 2) were having lower uric acid than stages 3 and 4. The authors postulated that antioxidant properties of UA decreased with increasing severity of the disease, and further exacerbations.
Nick ME et al., in their cross-sectional study on 136 smokers with normal lung function and 367 smokers with COPD observed a statistically significant negative association between serum UA (p< 0.002) and glutathione (p=0.03) with severity and exacerbation of COPD, respectively. Plasma ascorbate was not associated with any COPD phenotypes [20].
The Takahata study (N=2917, age ≥ 40 years, both males and females) reported significantly higher serum UA in males than females (5.8±1.3 vs. 4.5±1.1 mg/dl, respectively, p<0.001) [17]. The respiratory functions {forced vital capacity (r= -0.130, p< 0.001) and forced expiratory volume 1 (r=-0.118, p<0.001)} were inversely related to serum UA level among females, but not in males (p> 0.05). These associations were independent of age, body mass index, smoking status, alcohol intake, blood pressure and HbA1C. Similarly, another study on 59 stable COPD subjects without co-morbid conditions also reported an inverse relationship (p<0.05) between serum UA to creatinine ratio (UA: Cr) and respiratory functions and level of dyspnoea [18]. Another study from Japan validated the utility of UA to creatinine ratio in 91 COPD subjects (24 died of acute exacerbation; follow-up period; 31 months). UA: Cr ratio was increased in non survivors in comparison to survivor. This ratio was negatively correlated with lowest oxyhemoglobin saturation (r=-0.32, p<0.01) and independently related to mortality. Mortality was higher among patients with higher UA: Cr than those of low value (p<0.01) [24].
We observed significantly higher serum uric acid level in COPD cases with increased duration of the disease (p<0.05). COPD cases with more than 10 years’ duration had highest level of UA than those with <5 years and 6-10 years [Table/Fig-2,3]. This might be explained by the fact that as duration of the disease increases, lung function decreases leading to tissue hypoxia, inflammation, tissue destruction and increased uric acid production; which may further progress to systemic inflammatory disease [13].
Since UA is a product of hypoxia induced tissue destruction, it is postulated that oxygen and/or ventilation therapy might be useful [24,27]. There has been paucity of literature which highlights the relationship of serum UA levels in COPD subjects receiving such therapy. Sato N et al., analysed UA: Cr ratio among 91 outpatient COPD subjects receiving Home Oxygen Therapy (HOT) [24]. Twenty four patients died of acute exacerbation of COPD (mean follow up duration=31 months). Interestingly, percent changes in UA: Cr ratio (delta UA: Cr ratio) increased in non survivors than those who survived. There was inverse correlation between UA: Cr ratio and the nadir of oxyhemoglobin saturation (r = -0.32, p<0.01); and was found to be an independent predictor of mortality [24]. Another study evaluated the role of UA as a marker of acute exacerbation in patients with chronic respiratory failure treated with Non Invasive Positive Pressure Ventilation (NPPV) [27]. There was statistically insignificant decrease in UA: Cr ratio among 18/29 subjects following NPPV (p=0.0688). There was no significant association between change in UA: Cr ratio and that of PaO2 and PaCO2 after NPPV. Decrease UA: Cr ratio was significantly associated with fewer exacerbators (p=0.0021) [27]. Therefore, more in depth prospective studies should be done to study the impact of oxygen therapy and NPPV on UA levels in COPD.
Limitation
In spite of some positive outcomes, our study had certain limitations. Cross-sectional and observational nature of the study with limited number of subjects was the major reason for not getting a statistically significant outcome to justify our findings. Secondly, the smaller duration of our study with lack of adequate follow up data further weakened our outcome. Application of stringent inclusion criteria prevented us to derive the implication of hyperuricaemia among COPD subjects who had cardiovascular and renal co-morbidities. Lack of correlation between hyperuricaemia and prior therapy was another limitation of our study.
Conclusion
Outcome from our study provides the possible evidence that serum uric acid may be useful in assessing disease severity, progression and further exacerbation in COPD subjects. Serum uric acid is a simple, noninvasive, relatively inexpensive and readily available routine laboratory test which can be used in risk stratification in patients with COPD. Impact of oxygen and/or ventilation therapy on serum uric acid levels in these subjects needs to be further explored in future larger prospective studies.
COPD; chronic obstructive pulmonary disease, N; number of cases and control, SD; standard deviation, #; Student t test.
COPD; chronic obstructive pulmonary disease, N; number of cases, UA; uric acid, SD; standard deviation, SE; standard error, CI; confidence interval.
COPD; chronic obstructive pulmonary disease, GOLD; Global Initiative for Obstructive Lung Disease, N; number of cases, UA; uric acid, SD; standard deviation.
Abbreviations: UK; United Kingdom, COPD; chronic obstructive pulmonary disease, UA; uric acid, AECOPD; acute exacerbation of COPD, FVC; forced expiratory volume, FEV1; forced expiratory volume, GOLD; Global Initiative for Obstructive Lung Disease, N; number of cases, ICU; intensive care unit, GERD; gastroesophageal reflux disease.