Epilepsy is a heterogeneous neurological disorder affecting significant proportion of the population (~4%) at some time in life [1]. GGE {erstwhile known as Idiopathic Generalized Epilepsy (IGE)} accounts for one-third of all epilepsy cases [2], characterised by alone or combination of generalized seizures (absence, myoclonus and tonic-clonic seizures) with Electroencephalogram (EEG) hallmark of paroxysms of generalized spike-wave. Oxidative stress, defined as a persistent imbalance between the production of ROS and antioxidant defences [3], often results in irreversible cellular damage. Oxidative stress resulting from excessive generation of ROS has been implicated in the initiation as well as the progression of epilepsy [4] and also in the pathogenesis of many other neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease [5]. The ROS generated during normal cellular metabolism can be scavenged by enzymatic antioxidants namely SOD, GPx, CAT, or non-enzymatic antioxidants mainly vitamin E and reduced form of Glutathione (GSH) antioxidant defence mechanisms. However, oxidative stress results either due to increased ROS production or decreased antioxidant systems or both [6].
Accumulating evidence suggests that the chronic usage of AEDs such as valproic acid, phenytoin and carbamazepine may also increase free radical generation followed by oxidative damage in neurons [7].
Lipid peroxidation has been associated with pathological mechanisms involved in various neurological and neurodegenerative diseases [8]. Neurons are more predisposed to damaging effect of oxidative stress, due to the high concentration of Polyunsaturated Fatty Acid (PUFA) that is susceptible to lipid peroxidation [9].
NO is a signaling molecule in the Central Nervous System (CNS) and reacts with excess ROS to generate Reactive Nitrogen Species (RNS) like peroxynitrite, which is a powerful oxidizing agent that can cause lipid peroxidation, [10] thus contributing to free radical-mediated damage.
Although the role of free radicals has gained importance in neurochemical research, very few studies have been published on the role of antioxidants in GGE. Further, the relation between the antioxidant enzymes and different GGE types has not been reported. Hence, the present study was designed with an aim to evaluate oxidative stress markers, antioxidant enzymes in GGE patients, and to know the extent of oxidative stress induced by AEDs with the time duration of treatment.
Materials and Methods
Three hundred and ten, GGE diagnosed patients (male: female = 203:107), who were on AED treatment (n = 235), and 75 untreated patients (male:female = 49:26) along with 310 age and sex matched healthy controls were recruited for the present study from Nizam’s Institute of Medical Sciences, Hyderabad, India, from October, 2012 to December, 2014 with their written informed consent. The present study was approved by Ethical Committee of the “Institute of Genetics and Hospital for Genetic Diseases” as well as the study hospital. The clinical diagnosis was made according to admitted criteria by International League Against Epilepsy (ILAE) [12] on the basis of age of onset, type of seizures and EEG findings. In addition, patient’s Magnetic Resonance Imaging (MRI) of the brain was normal. The demographic characteristics including age, gender, age of onset of the disease, duration of the disease, and family history were collected using a structured questionnaire. The information about seizure type, its duration, frequency and AED medical history was also collected. Patients having diabetes mellitus, hypertension, acute, chronic renal failure and other metabolic disorders that are known to cause oxidative stress, were excluded from the study. Also, patients with smoking, tobacco chewing and alcohol habit were also excluded.
As IGE has an age related onset, we have divided the all IGE patients into “classical” IGE, with an onset before 18 years of age and adult onset IGE, epilepsy of later onset i.e., after 18 years of age on the basis of published reports [13] and the concept in the International Classification [12]. Again the patients were categorised into three groups depending on the time duration of AEDs (phenytoin, carbamazepine, sodium valproate etc.,) treatment (≤1 year, 1-≤5 years and >5 years).
About, 8 ml of venous blood was collected in plain sterile tubes from all the subjects, in case of patients blood was collected within 24 hours from the onset of seizure attack. A part of it was transferred to sterile Ethylenediaminetetraacetic Acid (EDTA) coated vacutainers for the estimation of erythrocyte SOD, GPx, CAT and plasma TAC. Remaining sample was transferred to sterile silicon tubes without addition of anticoagulants and centrifuged at 2000 rpm for 20 minutes to estimate, NO and MDA. Serum NO levels (nitrite and nitrate) (mmol/mL) were determined according to the method based on the diazotisation of sulfanilic acid by NO at acid pH and subsequent coupling to N-(1- naphthyl-ethylenediamine) [14]. MDA levels (nmol/mL) were measured by Buege JA and Aust SD, method using Thiobarbituric Acid (TBA) [15] and TAC by Ferric Reducing Ability of Plasma (FRAP) assay [16]. The erythrocyte GPx activity was estimated by Rotruck JT et al., method [17], SOD by Marklund SL and Marklund G method [18], CAT by Aebi H method [19].
Statistical Analysis
The difference in the levels of oxidative stress markers and activities of antioxidant enzymes between patients and controls was evaluated by Student’s t-test whereas, significance among patient subgroups who were on AEDs (such as phenytoin, carbamazepine, sodium valproate etc.) treatment for different time periods (≤1 year, 1-≤5 years and >5 years) were analysed by one-way Analysis of Variance (ANOVA) and the p-value of <0.05 was considered to be significant.
Results
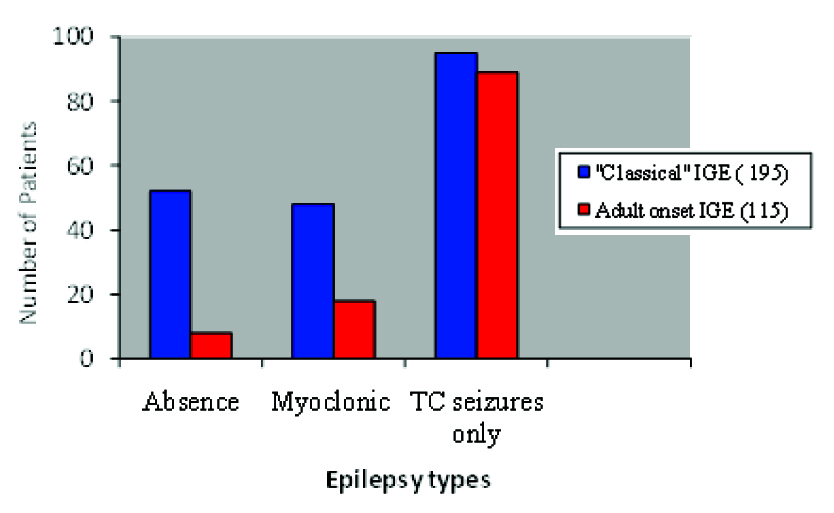
Out of 310 patients diagnosed with GGE, 195 (62.9%) patients indicating the majority had the onset before the age of 18 years (classical IGE) whereas, remaining 115 (37.1%) cases had the onset after 18 years of age (adult onset IGE). There was a higher proportion of females, 101(51.8%) in <18 years group (classical IGE), and similarly males, 64 (55.7%) in >18 years group (adult onset IGE). There was no significant difference in the proportions with a family history of epilepsy between “classical” group, 40 (20.5%) and “adult onset” group, 29 (25.2%). In both the age groups, the tonic-clonic variety of seizures and epilepsy types were more common. Similarly, only TC seizures epilepsy type was predominantly observed in 95 patients (48.7%) and 89 patients (77.4%) respectively in both “classical” as well as adult onset IGE groups [Table/Fig-1]. Division of patients in both classical and adult onset IGE was done [Table/Fig-2]. On the basis of seizure type, the patients with adult onset IGE were divided into three groups. Those with absence of tonic–clonic seizures were defined as “adult onset absence epilepsy”; the combination of myoclonic jerks and tonic–clonic seizures was defined as “adult onset myoclonic epilepsy” and patients with only tonic–clonic seizures were classified as “adult onset tonic–clonic epilepsy” [20].
Comparison of characteristics of patients with classical” and adult onset idiopathic generalized epilepsy.
| Variables | Age of onset <18 years (n = 195) | Age of onset >18 years (n = 115) |
|---|
| SexMaleFemale | 94 (48.2)101 (51.8) | 64 (55.7)51 (44.3) |
| Family History | 40 (20.5) | 29 (25.2) |
| Seizure typesAbsenceMyoclonusTonic clonic | 71 (36.4)36 (17.9)181 (92.8) | 3 (2.6)92 (80)112 (97.3) |
| Epilepsy typesAbsenceMyoclonusTonic clonic | 52 (26.7)48 (24.6)95 (48.7) | 8 (6.9)18 (15.7)89 (77.4) |
Note: Values are n (%) unless stated.
On the basis of predominant seizure type and age of onset, the international classification recognizes four IGE sub-syndromes [12] viz., Absence-Absence epilepsy, includes cases of both childhood absence epilepsy (CAE) and juvenile absence epilepsy (JAE); Myoclonic- Myoclonic epilepsy, includes juvenile myoclonic epilepsy (JME); TC- Tonic-clonic seizures, includes idiopathic generalized epilepsy and generalized tonic-clonic seizures on awakening (IGE+GTCS on awakening)
Different epilepsy types of “classical” and adult onset IGE with number of patients.
The levels of serum NO, MDA, plasma TAC, activities of erythrocyte SOD, GPx and CAT in IGE patients and controls have been given in [Table/Fig-3].
The serum MDA, NO, plasma TAC and erythrocyte antioxidant enzyme activities in GGE patients and controls.
| Parameters | Controls (n = 310) | Patients (n = 310) | p-value |
|---|
| NO (μmol/mL) | 5.03 ± 1.27 | 8.55 ± 2.0 | <0.0001* |
| MDA (nmol/mL) | 2.42 ± 0.87 | 4.39 ± 1.65 | <0.0001* |
| SOD (U/gHb) | 843 ± 95.2 | 606 ± 88 | <0.0001* |
| CAT (k/ml) | 9.39 ± 1.33 | 5.7 ± 1.24 | <0.0001* |
| GPx (U/gHb) | 76.7 ± 11.7 | 53.8 ± 8.85 | <0.0001* |
| TAC (μmol/L) | 8.58 ± 1.36 | 5.47 ± 1.23 | <0.0001* |
Note: p-value <0.05* is significant
Significantly higher levels of serum NO, MDA and low levels of plasma TAC were observed in patients (8.55±2.0 μmol/mL; 4.39±1.65 nmol/mL and 5.47 ±1.23 μmol/L) as compared to controls (5.03±1.27, 2.42±0.87 and 8.58 ± 1.36) (p<0.001) whereas activities of SOD, CAT and GPx were found to be significantly low in patients when compared to the control group (606±88 U/g Hb, 5.7±1.24 k/mL, 53.8±8.85 U/gHb and 843±95.2, 9.39±1.33, 76.7±11.7 respectively) (p<0.001). The levels of oxidative markers and antioxidant enzyme activities in IGE patients with AED treatment duration (≤1 year, 1-≤5 years and >5 years) have been shown in [Table/Fig-4].
Levels of serum oxidative markers and activities of antioxidant enzymes in IGE patients with different time duration of AED treatment.
| Parameters | 0=1 year(n = 54) | 1 = 5 years(n = 75) | >5 years(n = 106) | p-value |
|---|
| NO (μmol/mL) | 8.1 ± 1.59 | 8.42 ± 2.02 | 8.98 ± 2.14 | 0.02* |
| MDA (nmol/mL) | 4.1 ± 1.34 | 4.39 ± 1.68 | 4.87 ± 1.77 | 0.01* |
| SOD (U/gHb) | 622 ± 92.6 | 606 ± 88.7 | 573 ± 67.3 | 0.001* |
| CAT (k/ml) | 6.11 ± 1.16 | 5.69 ± 1.26 | 5.49 ± 1.22 | 0.01* |
| GPx (U/gHb) | 54.4 ± 8.68 | 53.6 ± 8.84 | 53.1 ± 8.99 | 0.6 |
| TAC (μmol/L) | 5.69 ± 1.14 | 5.46 ± 1.24 | 5. 2 ± 1.25 | 0.05* |
Note: p-value <0.05* is significant
0-≤1 year - Patients who were on AED treatment for less than 1 year
1-≤5 years- Patients who were on AED treatment for 1-5 years duration
>5 years- Patients who were on AED treatment for more than 5 years
Statistically significant difference in the levels of NO, MDA, activities of SOD, CAT and TAC were observed among patient subgroups who were on AEDs treatment for different time periods (p = 0.02, p = 0.01, p = 0.001, p = 0.01, p = 0.05 respectively) whereas no significant difference was noted in the activity of GPx between these subgroups. Finally, an attempt was made to compare the oxidative markers, antioxidant enzymes in IGE treated patients (AEDs) with untreated patients and the results have been depicted in [Table/Fig-5].
Comparison between oxidative stress markers, antioxidant enzyme activities in untreated and treated (with AEDs) patients of GGE.
| Variable | Treated Patients (n = 235) | Untreated Patients (n = 75) | p-value |
|---|
| NO (μmol/mL) | 8.55 ± 2.0 | 7.21 ± 1.86 | <0.0001* |
| MDA (nmol/mL) | 4.31 ± 1.65 | 3.82 ± 1.38 | 0.02* |
| SOD (U/gHb) | 606 ± 88 | 676.9 ± 82.1 | <0.0001* |
| CAT (k/ml) | 5.7 ± 1.24 | 6.21 ± 1.1 | 0.001* |
| GPx (U/gHb) | 53.8 ± 8.85 | 55.9 ± 6.81 | <0.06 |
| TAC (μmol/L) | 5.47 ± 1.23 | 5.82 ± 0.96 | <0.02* |
Note: p-value <0.05* is significant
Significantly increased levels of NO, MDA, decreased activities of antioxidant enzymes (SOD, CAT) and TAC were observed in IGE treated patients compared to untreated patients (p<0.001, p = 0.02, p<0.001, p = 0.001, and p = 0.02 respectively).
Discussion
Many studies have suggested that the serum electrolytes, trace elements and lipid peroxidation, due to oxidative stress are causally involved in some epilepsy types and their seizure recurrence [21]. The brain contains high concentration of PUFA that are more prone to lipid peroxidation. Hence, the assessment of MDA levels in biological materials can be used as an important indicator of lipid peroxidation in vitro and in vivo for various diseases. In the present study, we found significantly higher levels of MDA in IGE patients compared to controls. Our results are in accordance with many previous studies where a higher level of MDA has been reported to be associated with epilepsy [22-24]. Conversely, no difference [25] or significantly decreased [26] levels of MDA have been reported in epilepsy patients. It was also found that significantly increased MDA levels in treated (with AEDs) compared to untreated IGE patients suggest that the additional oxidative stress was induced by AEDs, thereby causing seizure recurrence as well as drug intolerance.
It has been suggested that in CNS, NO plays a significant role in epilepsy and epileptogenesis because it acts as a secondary messenger, neuromodulator and neurotransmitter [27]. In the present study, NO levels were found to be significantly high in patients when compared to controls. Our results are in agreement with many other studies, where significantly elevated NO levels have been reported to be associated with the disease [25,28]. Similarly, significantly elevated serum NO levels were observed in treated patients compared to untreated patients denoting that additional oxidative stress was induced by AEDs which results in seizure recurrence and drug intractability.
The superoxide dismutases play a crucial role in eliminating superoxide anion radicals (O2•−) generated from extracellular stimulants, including ionizing radiation and oxidative insults together with produced in mitochondrial matrix [29]. In the present study, we found a significant decrease in SOD activity in patients as compared to controls. Our results are in accordance with earlier studies where a significantly lower activity has been found in patients with GGE [30]. Conversely, increased levels of SOD have been noted in drug naive patients with epilepsy [22] and unchanged SOD levels in other studies [28]. In order to maintain the proper functioning of Cu-Zn SOD1 enzyme, the Cu-Zn ratio is important rather than absolute amount of Cu or Zn alone [31]. In our previous study, we found significantly low levels of serum zinc and increased Cu/Zn ratio in GGE as well as in IGE patients [32]. In addition, significantly lower activities of SOD were observed in treated patients of epilepsy compared to untreated patients suggesting that additional oxidative stress induced by AEDs may interfere with the pathogenesis of epilepsy. Hence, in addition to high Cu/Zn ratio, low levels of Zn and SOD reflects SOD function which may lead to ineffectual scavenging of ROS and resulting in seizure recurrence.
Our study showed significant lower activities of CAT in GGE patients compared to controls. Our results are in agreement with the earlier studies, where significant low levels were claimed in patients compared to controls [22,33]. Also, the CAT levels were found to be significantly low in treated patients compared to untreated patients. The low levels of CAT can be attributed to inactivation of enzyme by superoxide anions and thereby inducing additional oxidative stress indirectly which results in seizure promotion and drug intractability as well.
In the present study, the erythrocyte GPx activity was found to be significantly low in patients when compared to controls. Our results are in accordance with Saad K et al., who reported both Selenium (Se) and GPx activity to be reduced in children with intractable seizures [34]. Significantly lower activity of GPx was observed in treated patients as compared to untreated patients suggest that increased oxidative stress induced by AEDs may interfere in the pathogenesis of seizures and causing unresponsiveness to AEDs.
TAC can be considered as a cumulative index of complete antioxidant capacity. In the present study, the level of TAC were significantly decreased in IGE patients compared to controls and in treated patients compared to untreated patients suggesting that lower antioxidant enzymes activity may also contribute to the ongoing process of epileptogenesis.
Accumulating evidence suggests that the chronic usage of first-choice AEDs such as valproic acid, phenytoin and carbamazepine increases free radical formation followed by oxidative damage in neurons [7]. But AEDs such as zonisamide and topiramate have antioxidant effects by scavenging hydroxyl radicals and NO in experimental conditions [4]. The possible mechanism behind oxidative stress induced by AEDs is that these drugs are metabolized to reactive epoxide intermediates, which may covalently bind to biomolecules such as lipids, proteins etc., and induce structural and functional impairments of the cell rather than having a neuroprotective effect [35]. In the present study, a significant difference in the levels of NO, MDA, decreased activity of SOD and CAT were noticed between the patient subgroups of AED treatment duration viz., ≤1 year, 1-≤5 years and >5 years. A significant decrease in the activities of SOD, CAT and TAC and increased level of NO and MDA in treated patients compared to untreated patients of GGE signify the role of AEDs in the induction of oxidative stress, utilization of antioxidant enzymes and thereby reducing the antioxidant capacity that may result in seizure recurrence and drug unresponsiveness. Our results are in accordance with previous study in which the decreased activities of antioxidant enzymes and also a positive effect of AEDs on oxidative stress were noticed [36]. Our results suggest that chronic usage of AEDs may trigger oxygen-dependent tissue injury that lead to additional oxidative stress, which may be implicated in the seizure generation, progression and drug intractability.
Limitation
Further studies are required to establish the role of individual AEDs in the development of oxidative stress. The clinical relevance of the study has to be evaluated.
Conclusion
The present study demonstrates that the epilepsy seems to cause the elevation of NO and MDA levels that reflects the oxidative stress in GGE patients. The pro-oxidative effects of AEDs might lead to additional oxidative stress, increased seizure activity resulting in loss of their efficiency and drug intractability. The concomitant use of antioxidants along with conventional AEDs could have a beneficial effect.
Note: Values are n (%) unless stated.
On the basis of predominant seizure type and age of onset, the international classification recognizes four IGE sub-syndromes [12] viz., Absence-Absence epilepsy, includes cases of both childhood absence epilepsy (CAE) and juvenile absence epilepsy (JAE); Myoclonic- Myoclonic epilepsy, includes juvenile myoclonic epilepsy (JME); TC- Tonic-clonic seizures, includes idiopathic generalized epilepsy and generalized tonic-clonic seizures on awakening (IGE+GTCS on awakening)
Note: p-value <0.05* is significant
Note: p-value <0.05* is significant
0-≤1 year - Patients who were on AED treatment for less than 1 year
1-≤5 years- Patients who were on AED treatment for 1-5 years duration
>5 years- Patients who were on AED treatment for more than 5 years
Note: p-value <0.05* is significant