The increasing demand to attain a picture perfect smile has led to a rising trend among individuals seeking orthodontic treatment, with aesthetics forming the main reason for seeking the treatment [1]. Unfortunately, one of the possible undesirable consequences of orthodontic therapy is the occurrence of white spot lesions. Despite the advances in orthodontic materials and techniques in recent years, nearly 50% of orthodontic patients exhibit clinically visible enamel demineralization around orthodontic brackets [2]. There are different methods which can decrease or prevent white spot lesions: improving oral hygiene, modifying diet (low carbohydrate) and treating with topical fluoride. However, most of these methods are patient dependent and compliance is only seen in 13% of the patients [3]. Thus, attempts have been made to use compliance-free methods.
It has been seen that fluoride varnish application results in 44.3% decrease in enamel demineralization [4]. However, repeated varnish applications may lead to the temporary discoloration of the teeth and gingival tissue and increase costs to the patient and chair time to the clinician [5].
Thus, the present study was conducted in vivo for evaluation of enamel topography and surface microhardness after surface treatment with CO2 laser, Er,Cr:YSGG laser and 5% sodium fluoride varnish.
Materials and Methods
The present in vivo double blind intervention study was conducted from March 2015 onwards till March 2016 on patients undergoing fixed orthodontic treatment in the Department of Orthodontics and Dentofacial orthopaedics at Maulana Azad Institute of Dental Sciences, New Delhi, India. Approval was taken from the Institutional Ethical Committee.
Keeping the power of the study at 80% and the probability of type I error at 5%, the interventional study was carried out on 100 premolars which were divided into five groups, each comprising of 20 teeth. The control group was formed by 20 sound enamel samples which were devoid of any surface malformations, cracks and discoloration and the rest 80 premolars were the interventional group. The interventional group had patients in the age group of 13 to 25 years requiring fixed orthodontic treatment with extraction of all 4 first premolars.
The inclusion criteria of the samples were fully erupted first premolars, low caries risk as assessed by caries risk assessment tool given by American dental association [16], premolars devoid of any evident enamel lesions or restoration on the buccal surfaces of teeth. Patients with dental fluorosis or any systemic disease affecting the formation of enamel were excluded from the study. All the subjects who fulfilled the inclusion criteria and gave a written consent for the study were included.
Group 0 served as control group and provided the baseline enamel topography and surface microhardness of sound enamel where no bonding was done and no surface treatment was given.
The interventional group comprised of Groups I to IV.
Stainless steel orthodontic brackets were bonded to the buccal surface of the first premolars included in the study. For this reason, the buccal surfaces of the teeth were etched for 15 seconds with 37% phosphoric acid. Transbond XT primer was painted on the etched surfaces with an applicator tip. The primer was cured for 10 seconds as per the manufacturer’s instructions. Transbond XT adhesive was applied at the back of the bracket and pressed firmly against the tooth surface and excess resin was removed with an explorer and then polymerized with the LED light-curing system. After bonding, random allocation of these teeth via the lottery method was done into the four groups (based on the surface treatment of enamel) to remove bias between maxillary and mandibular arches.
The surface treatment viz., CO2 laser, Er,Cr:YSGG laser and 5% sodium fluoride varnish was given once immediately after bonding on all the aspects of buccal surface of the tooth around the orthodontic bracket.
In Group I, the enamel surface of the premolar was treated with CO2 laser (FUTURA R2 CO2 Fractional laser, Inc. USA) having wavelength of 10.6 µm, 1 watt power, 1 mm of beam diameter, 20 HZ frequency for 12 seconds.
In Group II, the enamel surface of the premolar was treated with Er,Cr:YSGG laser (BIOLASE Waterlase® YSGG, BIOLASE® Technology, Inc. USA) with specifications of 2.78 µm wavelength, 0.75 watt power, 1 mm beam diameter, 20 HZ frequency for 20 seconds.
For laser application, all the safety measures as described under ANSI 136.1 (American National Standard Institute) were assured. Once the laser was set as per the specifications, the laser tip was held at an approximate distance of 1 mm from the tooth surface and moved uniformly starting from the bracket- tooth interface extending out towards the periphery of the tooth all around the bracket.
In Group III, the enamel surface of the premolar was treated with 5% sodium fluoride varnish (VOCO. Profluorid, Inc. Germany). Application of 5% sodium fluoride varnish was accomplished as per ADA guidelines for Fluoride application.
In Group IV, the bonded premolar received no surface treatment.
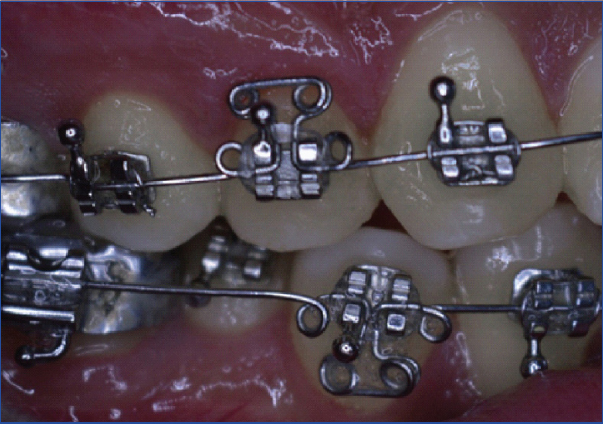
A modified T loop was fabricated with 0.016” stainless steel continuous archwire. The T loop was incorporated with a helix of 1 mm on both ends of the arm of the loop. Two helices 1 mm in diameter were incorporated onto the arch wire at a distance of 3 mm from the vertical arm of T loop on each side. The T loop was engaged using an elastomeric ligature to increase plaque accumulation [Table/Fig-1].
T loop engaged using an elastomeric ligature to increase plaque accumulation.
After two months, the premolars were extracted and the teeth were disinfected by keeping them in 10% formalin for 48 hours as per OSHA regulations [17] and then stored at room temperature in distilled water till the time of SEM analysis and microhardness testing. For the SEM analysis, the samples were coated with 40 nm to 60 nm of gold using a sputter coater (Palaron-SC 7640, United Kingdom) and then observed in the microscope (Carl Zeiss EVO 40) at the magnification of 2000X [18]. For the purpose of testing the enamel microhardness of all the samples, Vickers hardness testing machine (Microhardness testing machine, MITUTOYO YO, MVK-1) was used. A constant load of 100 g for 10 seconds was applied perpendicular to the enamel surface with the diamond intender to create indentations at three points that were measured using a computerized program. An average of the three readings obtained for per sample was taken.
Statistical Analysis
All statistical analysis was performed using SPSS 18.0 software. Descriptive statistics including the mean and standard deviations for enamel microhardness were calculated for each of the five groups. One-way analysis of variance (ANOVA) was used to determine whether significant differences existed between the various groups. Post-hoc multiple comparison test with Bonferroni correction was used to identify which of the groups were significantly different.
Results
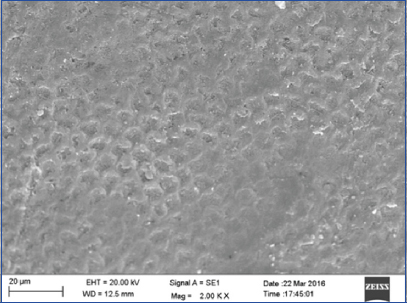
SEM evaluation of Group 0 revealed circumferentially arranged enamel rods filled with inter rod material producing a typical keyhole appearance [Table/Fig-2].
SEM photograph of Group 0 (control) exhibited circumferentially arranged enamel rods filled with inter rod material on the surface (magnification 2000X).
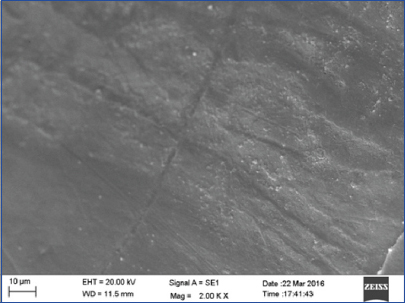
Evaluation of Group I showed a homogenous enamel surface with fine cracks and fissures over the enamel surface [Table/Fig-3].
SEM photograph of Group I (CO2 laser) showed a melted enamel appearance with fine cracks and fissures (magnification 2000X).
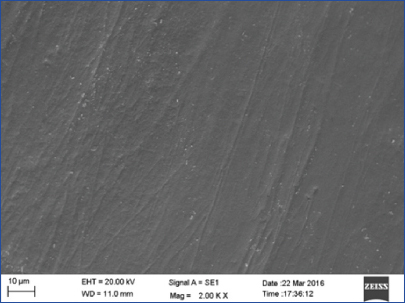
Group II showed melting of enamel rods giving rise to a glossy enamel surface with well coalesced enamel rods. Porous structure of enamel was lost giving rise to a smooth surface [Table/Fig-4].
SEM photograph of Group II (Er,Cr:YSGG laser) showed a smooth, homogenous enamel surface with well coalesced enamel rods (magnification 2000X).
Evaluation of Group III showed streaks of particle deposition of less than 1 µm in size on the enamel surface. Areas of slight erosions and porosities were also visible near the bracket tooth interface [Table/Fig-5].
SEM photograph of Group III (5% Sodium fluoride varnish) showed areas of slight erosions near the bracket tooth interface. (at magnification 2000X).
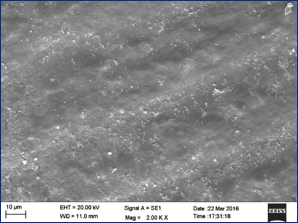
The SEM evaluation of Group IV revealed areas of stripped enamel revealing the enamel rods vividly. Areas of isolated cavitations caused by erosion of the intact enamel surface showed deposition of bacterial colonies [Table/Fig-6].
SEM photograph of Group IV (no surface treatment) presented areas of stripped enamel revealing the enamel rods which were exposed vividly. Enhanced enamel porosities with loss of prismatic structure were appreciated (at magnification 2000X) (marked with red arrow).
Results of surface microhardness were obtained using Vickers microhardness testing machine. [Table/Fig-7] depicts the mean, minimum and maximum values of surface microhardness (VHN) and standard deviations for each group.
Descriptive statistics of enamel microhardness acquired using Vickers Microhardness Test (VHN).
| Groups | N | Mean | Std. deviation | Minimum | Maximum |
|---|
| Group 0 control | 20 | 125.50 | 9.75 | 110 | 138 |
| Group I CO2laser | 20 | 259.83 | 14.02 | 236 | 286 |
| Group II Er,Cr:YSGG laser | 20 | 174.91 | 15.30 | 149.33 | 196.33 |
| Group III 5% NaF varnish | 20 | 117.56 | 8.63 | 101 | 129.67 |
| Group IV No surface treatment | 20 | 103.36 | 12.48 | 73 | 134 |
The highest microhardness value was exhibited by Group I followed by Group II, Group 0 and Group III. The least microhardness value was observed in Group IV.
Level of significance of mean microhardness within groups and between groups was analysed using one-way ANOVA test. There was a significant difference of mean microhardness among five groups with significance of p<0.001 [Table/Fig-8]. Post-Hoc test with Bonferroni correction was done for comparison of mean microhardness between five groups [Table/Fig-9]. Significant difference was observed between the mean microhardness (VHN) of Group I (CO2 Laser), Group II (Er,Cr:YSGG Laser), Group III (5% NaF varnish), Group IV (no surface treatment) and Group 0 (control) with p<0.001. A significant difference of p<0.001 was observed while comparing Group I vs II, III, IV, 0 and Group II vs III, IV, 0. However, significant difference while comparing Group III vs IV was p=0.005 and difference between the mean microhardness of Group 0 vs Group III was non significant
Level of significance of enamel microhardness using one-way ANOVA test.
| Groups | Sum of squares | df | Mean square | F | p-value |
|---|
| Between Groups | 324429.340 | 4 | 81107.3350 | 529.695 | <0.001 |
| Within Groups | 14546.4380 | 95 | 153.1204 | | >0.05 |
| Total | 338975.7780 | 99 | | | |
Intergroup comparison of mean microhardness using a post-hoc test.
| Groups | Mean difference | p-value |
|---|
| Group I vs 0 | -133.5 | <0.001*** |
| Group I vs II | 84.45 | <0.001*** |
| Group I vs III | 141.80 | <0.001*** |
| Group I vs IV | 156.00 | <0.001*** |
| Group II vs 0 | -49.15 | <0.001*** |
| Group II vs III | 57.35 | <0.001*** |
| Group II vs IV | 71.55 | <0.001*** |
| Group III vs 0 | 7.800 | 0.441 (NS) |
| Group III vs IV | 14.20 | 0.005** |
| Group IV vs 0 | 23.05 | <0.001*** |
Level of significance <0.001,
Level of significance <0.01, NS- not significant.
Discussion
Orthodontic treatment usually lasts for two years during which almost 50% of patients exhibit clinically visible white spot lesions [2]. Studies state that the rate of decalcification in orthodontic patients especially teenagers is higher than those without orthodontic treatment [19-22]. Post orthodontic treatment, white spot lesions are shown to decrease during the first and second years after debonding. However, areas of demineralization will still remain on the teeth for up to five years after orthodontic treatment and thus posing a cosmetic problem [23].
Some Indian studies like that of Malik A et al., and Chand BR et al., have evaluated the efficiency of lasers and fluoride and their combination in preventing enamel demineralization [24,25]. Although these studies have found positive results in regard to the laser group and fluoride+ laser combination group. However, the limitation in most of these studies was that they were performed in vitro.
Hence, the present in vivo study was undertaken with the intention that greater clarity could be achieved regarding which method of surface treatment could provide us with the best results in decreasing demineralization around orthodontic brackets. In the present study, the three surface treatment agents employed were CO2 laser, Er,Cr:YSGG laser and 5% sodium fluoride varnish. First premolars were chosen as sample teeth for our study as not only they are most commonly extracted teeth for orthodontic treatment but also most commonly affected by white spot lesions [26,27]. Since the increased incidence of white spot lesions is due to greater number of stagnation areas owing to fixed appliances, in addition to the bonded appliance, a loop was designed to increase the plaque accumulating surface. Regarding the time required for demineralization, it has been preferentially studied and proven that demineralization of enamel around brackets can be an extremely rapid process with lesions of significant depth (75 µm) can developin four weeks, a shorter time than many orthodontic appointment intervals of 6 to 10 weeks [1,10,20]. In the present study, the first premolars were extracted after a period of two months (within which alignment of the teeth was carried out using the 0.016” stainless steel continuous arch wire) and were later subjected for evaluation of the enamel surface by SEM and surface microhardness testing.
One of the surface treatment agents we used in the present study was CO2 laser. Featherstone JDB et al., examined the roles of wavelength (9.3 µm, 9.6 µm, 10.3 µm, or 10.6 µm) in the prevention of caries progression in vitro in enamel [28]. Inhibition of caries progression from 40% to 85% was achieved over the range of laser conditions tested with the optimal results produced by the wavelength of 10.6 µm. In Group I, CO2 laser was applied on to the enamel surface around the orthodontic bracket in vivo with the specifications (wavelength 10.6 µm, 1 watt power, 1 mm of beam diameter, 20 HZ frequency) for 12 seconds which according to the literature gave the maximum results in decreasing demineralization [15]. The SEM images revealed melting of enamel surface giving a homogenous appearance along with fine cracks and fissures distributed over the surface. The mean microhardness of enamel achieved after CO2 laser surface treatment was significantly higher than the control group as also observed in respective studies by Miresmaeili A et al., [27] and Strangler L et al., [29] wherein they observed that enamel samples treated with CO2 laser exhibited a higher value of microhardness as compared to enamel which did not receive any surface treatment. The change in the ultrastructure of enamel can be explained by the fact that surface alteration is achieved from the melting and the subsequent recrystallization of the enamel during cooling. This phenomenon could lead to formation of a resistant enamel surface produced by prominent chemical and mineral content changes [30]. These mineral and structural changes brought by the laser irradiation could be the possible reason that a significantly high microhardness value was achieved. As mentioned earlier, along with the melting of enamel surface, fine cracks and fissures could also be visualized which might act as retentive areas for plaque as also observed in other studies like that of Contreras-Bulnes R et al., [13] where in after surface treatment with CO2 laser (10.6 µm), SEM analysis of enamel surface revealed rough areas, craters and cracks. In contrast to this, there have been studies which do not report any unwanted appearance of cracks over the enamel surface after the application of CO2 laser and claim that a smooth morphology of enamel is obtained with CO2 laser treatment [10,14,15,27,31]. The differences observed in results obtained after application of CO2 laser in various studies could be due to non uniformity of methodology and laser parameters such as power, beam diameter, frequency and duration of exposure. This notion stresses on the fact that a wider range of understanding and evaluation of different parameters of CO2 laser is required to attain its maximum benefit in decreasing demineralization.
In Group II, the SEM evaluation revealed a homogenously melted, smooth and glossy looking enamel surface. No cracks and fissures were observed. Out of all the groups evaluated, this group presented with optimal microscopic alterations in the enamel surface showing coalescing of enamel rods making them impervious to acidic dissolution. A significantly higher microhardness for enamel was achieved as compared to the control group. These finding are well in accordance with studies by Geraldo-Martins VR et al., Hossain M et al., and Moslemi M et al., who in their individual studies reported a significant decrease in enamel demineralisation after surface treatment with Er,Cr;YSGG laser owing to the appearance of a smooth enamel surface devoid of any cracks [26,32,33].
The evaluation of enamel samples falling under Group III by both the SEM analysis and surface microhardness test revealed that demineralization had occurred around the orthodontic bracket in spite of varnish application. These findings were in accordance with the findings of Basdra EK et al., who used SEM to study the effect of fluoride releasing agents and found that maximum fluoride release was seen in the first 24 hours of application with a steady decline thereafter. They found fluoride release to be negligible after 90 days [34]. Our findings for fluoride varnish were also in accordance with the study conducted by Vivaldi Rodrigues G et al., where they evaluated the effect of varnish in limiting demineralization. They found no statistically significant difference between the mean enamel decalcification index for the control and experimental groups (fluoride varnish) [4].
Group IV samples which received no surface treatment showed significant decrease in microhardness as compared sound enamel. SEM view also showed a rough enamel surface with surface erosions and shallow cavitations with bacterial colonies deposited on them in agreement with the finding that demineralization starts as soon as four weeks after placement of the orthodontic appliance [10, 20].
Limitation
The present interventional study was a short term in vivo study wherein a single surface treatment by CO2 laser, Er,Cr:YSGG laser and 5% sodium fluoride varnish was done. Long term studies need to be carried out to assess the sustainability of the results obtained with a single application of surface treatment agents and whether multiple applications are required for efficiently preventing demineralization for the complete duration of orthodontic treatment.
CONCLUSIONS
According to our present investigation, it was concluded that both the CO2 laser and the Er,Cr:YSGG laser were successful in increasing the enamel surface microhardness whereas surface treatment with fluoride varnish did not significantly affect the microhardness of enamel. The SEM evaluation showed that surface treatment with CO2 laser created cracks and fissures on the surface which may act as retentive areas for plaque accumulation. It could also be stated that surface treatment with Er,Cr:YSGG laser causes a positive alteration of the enamel surface increasing its ability to resist demineralization with optimum microhardness.
***Level of significance <0.001,
**Level of significance <0.01, NS- not significant.