OSCC is one of the common oral malignancies that frequently presents with a clinical diagnostic challenge, particularly in early stages. The incidence of OSCC varies broadly in different parts of the world, ranging from 2-10 per 1,00,000 cases per year [1]. In India the incidence rate of OSCC is 12% in men and 8% in women among all the oral cancers [2]. Despite advances in treatment achieved, the prognosis of oral cancer still remains poor with low survival rate. Invasion and metastasis are the most important causes of cancer related morbidity and mortality and found to be the biological hallmarks of malignant tumours [3]. OSCC is an invasive lesion with a significant recurrence rate often metastasizing to regional cervical lymph nodes, where 40% of patients with oral cancer present with lymph node metastasis. Metastasis is a process that occurs in a sequence of proliferation of the neoplastic cell, neoangiogenesis, detachment of the cell from primary site followed by invasion into bloodstream creating a new microenvironment along with stroma and inflammatory cells. Due to host immune response very few neoplastic cells follow the metastatic pathway. Thus, metastasis represent an escape of malignant cells from tumour microenvironment created by itself that include deprivation of oxygen and nutrients, inflammation and immune system attacks [4].
Few factors that help Bcl-2 to prevent apoptosis are serum deprivation, heat shock protein and chemotherapeutic reagents via a similar pathway [9]. In OSCC, the common feature appreciated is inhibition of apoptosis.
Bcl-2 is expressed in basal layers in normal epithelium. Early phase of epithelial carcinogenesis is attributed to Bcl-2 protein over expression [5]. Staging of cancer is considered as one of the main factor that determines the prognosis of the patient. But about 15% to 34% of oral cancer does not manifest clinically [4]. However, TNM staging does not provide an accurate prognosis, as the tumours of similar stage can possess a different biological behaviour. To identify different risk groups with diverse biological behaviour, an identification of a reliable prognostic marker is a must. In recent years, multiparametric evaluation plays an important role in assessing the prognosis and disease free survival [10]. Hence, in this study we aimed to evaluate and correlate the Bcl-2 expression in patients with lymphnode metastasis and without metastasis of OSCC.
Materials and Methods
This retrospective study comprised of 30 samples with each 15 cases of metastatic and non-metastatic primary OSCC treated from 2010 to 2016 at Faculty of Dental Sciences, Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India. Cases with details of radical neck surgical treatment with required clinical and pathologic data with paraffin-embedded tumour specimens were considered.
Clinico-pathological parameters integrated in present study were age, gender, habits, tumour location, treatment, clinical staging. The diagnosis of these cases was confirmed by histopathological examination of primary tumour.
One set of sections of all the primary tumour were subjected for routine hematoxylin and eosin stain for the evaluation of histopathological parameters like pattern of invasion, lymphocytic infiltration and histopathological grading of primary tumour. Other set of tumour tissue sections were stained with clone 100/D5 Bcl-2 antibody by immunohistochemistry. Lymphnode was taken as positive control for the Bcl-2 staining.
Inclusion criteria: Individuals with clinical evidence and histopathologically diagnosed OSCC cases were included in the study.
Exclusion criteria: Recurrent cases were excluded.
Histopathological Analysis
Grading of OSCC was categorised according to Broder’s classification of grading system given in WHO guidelines (2005).
LI and POI at the tumour–host interface were evaluated according to Brandwein-Gensler M et al., [11].
LI was quantified into three types:
Type 1 Dense continuous rim of lymphoid tissue [Table/Fig-1a];
Photomicrographs of H&E stained sections showing types of lymphocytic infiltrate at tumour host interface: a) Dense continuous infiltrate (4X); b) Discontinuous or patchy infiltrate (4X); c) Minimal or scanty infiltrate (4X).
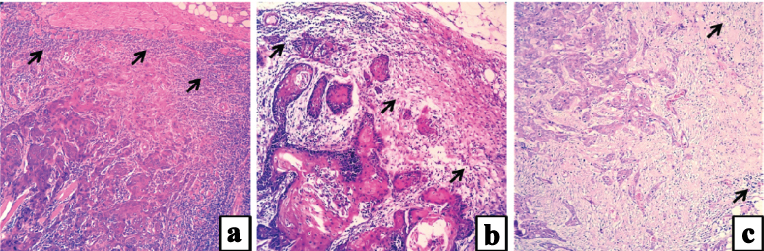
Type 2 Discontinuous lymphocytic infiltrate or dense lymphoid patches [Table/Fig-1b];
Type 3 Limited infiltrate or no lymphoid response [Table/Fig-1c].
POI was categorised into five types:
POI 1 Broad pushing front [Table/Fig-2a];
Photomicrographs of H&E stained sections showing Pattern of invasion at tumour host interface: a) Broad pushing front (10X); b) Finger-like pushing front (4X); c) Islands with >15 cells (10X); d) Islands with <15 cells (10X); e) Dispersed pattern (4X).
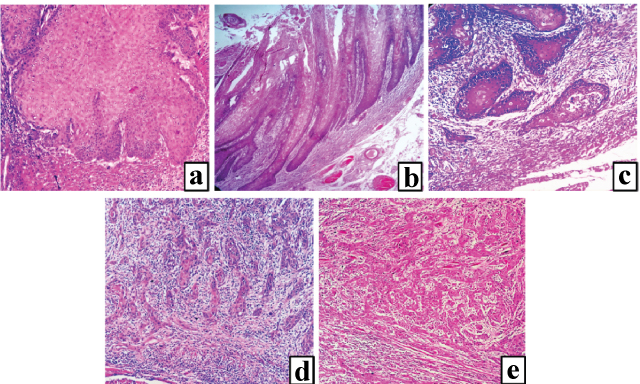
POI 2 Finger-like pushing front [Table/Fig-2b];
POI 3 Islands at the tumour-stromal interface with >15 cells per island [Table/Fig-2c];
POI 4 Strands or islands with <15 cells [Table/Fig-2d];
POI 5 Dispersed pattern [Table/Fig-2e].
Immuhistochemical Staining Procedure
4 µm thick sections from FFPE tissue blocks were mounted on poly-L-lysine-coated slides and were incubated at 60°C for one hour in hot air oven. After rinsing with three changes of xylene for deparaffinization, the sections were rehydrated with ethanol at different descending concentrations 100%, 95% and 70% for three minutes each and washed with distilled water. Antigen retrieval was done for 15-20 minutes using EDTA buffer at 90°C with pH 8 by using pressure cooker method of retrival. The slides were washed in tris-buffer solution for five minutes. To inactivate endogenous peroxidase, sections were incubated for five minutes in 3% H2O2 and then rinsed with tris-buffer solution. The slides were treated with 100/D5 Bcl-2 antibody at a dilution of 1:100 incubated for a period of one hour at room temperature, then washed using wash buffer for two minutes each. Mouse probe was used for 15 minutes. Immune complexes were treated with horseradish peroxidase polymer for 30 minutes and incubated at room temperature. After rinsing with PBS, the immunoreactivity was visualized by 3,3’-Diaminobenzidine (DAB) chromogen incubated for five minutes. The tissues were counterstained with hematoxylin. The slides were dehydrated and mounted with DPX.
Interpretation of IHC Staining
Antigen expressed cells were evaluated using a light microscope at magnifications of 100X and 400X. Brown staining of nuclear membrane and cytoplasm that suffices the positive control was considered as positive reactivity for test group. Absence of specific staining was observed in negative control tissue. All the sections were evaluated for distribution of Bcl-2 protein expression and staining intensity by two observers using the scoring criteria given by Sudha VM et al., and Suri C et al., [1,12]. In each case, distribution was evaluated based on the percentage of positively stained cells at a magnification of 100X. The intensity of stain was graded in comparison to the positive control.
Immunohistochemical Analysis of Expression of Bcl-2
The staining intensity (I) was graded using the following scoring criteria, 0-negative, 1-weakly positive, 2-moderately positive, 3-strongly positive.
Positive staining distribution (P) was graded as grade 1-(0-10%), grade 2-(10-30%), grade 3-(30-60%), grade 4-(>60%).
Statistical Analysis
Chi-square test was employed to draw the correlation between the study groups and parameters with a confidence interval of 95% and significance <0.05 (p).
Results
Study samples comprised of 30 patients. There were 19 females and 11 males with median age of 55 years. The intraoral sites included buccal mucosa: n=20, gingivo-buccal sulcus: n=4 and tongue: n=6. Pathological staging: Stage I, n=3 (10%); Stage II, n=6 (20%); Stage III, n=5 (16.7%); and Stage IV, n=16 (53.3%). All patients were treated with radical resection of the primary tumour with tumour free margins followed by radiotherapy and chemotherapy. 15 out of 30 patients were diagnosed with lymphnode metastasis (pN).
The expression of Bcl-2 protein was localized in the cytoplasm and nuclear membrane of tumour cells in a granular pattern. In the current study, overall positive rate of Bcl-2 staining was 30%.
The parameters used for analysis of the study groups were, lymphocytic infiltration, pattern of invasion, staining intensity and distribution.
Clinical Parameter
Based on the WHO TNM staging the, tumour size and regional lymphnode status were considered as parameters to study the difference between metastatic and non metastatic OSCC.
Tumour size: The study group did not show statistically significant difference with respect to the tumour size, where T1 was observed in 3 (10%) cases in both metastatic and non metastatic group. T2 in 4 (13.3%) of metastatic and 6 (20%) cases of non metastatic group. A total of 1 (3.3%) and 2 (6.7%) cases were in T3 stage. T4 was observed in 7 (23.4%) and 4 (13.3%) of the study groups respectively and there was no statistically significance (p=0.67) between the groups.
Nodal status: Out of 30 study samples, 15 (50%) were of non metastatic (No stage) Group and 2 (6.7%) cases were in N1 stage and 13 cases in N2 stage of metastatic group. Statistically significant difference of p=0.00001 was observed between metastatic and non metastatic group.
Histopathological Parameter
Lymphocytic Infiltration (LI): The lymphocytic infiltrate was categorised into three types where Type 1 was observed in 9 (30%) cases of metastatic and 7 (23.3%) cases of non-metastatic group, Type 2 in 3 (10%) cases of metastatic and 7 (23%) cases of non-metastatic group and Type 3 (10%) in 3 cases of metastatic and 1 (3%) case of non-metastatic group. The results did not show any statistical significance between metastatic and non-metastatic groups (p=0.24).
Pattern of Invasion (POI): Five types of POI were identified where POI 1 was observed in 1 (3%) case of metastatic and 3 (10%) cases of non-metastatic group, POI 2 was observed in 4 (13%) cases of non-metastatic group alone, POI 3 in 3 (10%) cases of metastatic and 2 (6.7%) case of non-metastatic group. POI 4 was observed in 9 (30%) cases of metastatic and 5 (16.7) cases of non-metastatic group, POI 5 in 2 (6.7%) cases of metastatic and 1 (3%) case of non-metastatic group. The results did not show any statistical significance between metastatic and non-metastatic groups (p=0.15).
Immunohistochemistry
Distribution of Bcl-2 protein expression among metastatic and non metastatic group of OSCC: Percentage of negative cases was comparatively high in both metastatic 11(36.6%) and non metastatic groups 9 (30%). Distribution of Bcl-2 staining was low in 4 (13.3%) cases of metastatic and 5(16.6%) cases of non-metastatic group. Only 1 (3%) case showed moderate expression that was statistically insignificant (p= 0.51).
Intensity of Bcl-2 staining in metastatic and non metastatic group of OSCC: Eight (26.6%) cases of metastatic OSCC showed negative staining intensity and 7 (23.3%) showed weakly positive staining intensity. Whereas, 9 (30%) cases of non-metastatic OSCC showed negative staining intensity, 5 (16.6%) cases showed weakly positive and 1 (3%) case showed moderately positive staining intensity. There was no significant difference between metastatic and non-metastatic group (p=0.49).
Discussion
Bcl-2 and related group of proteins comprise the Bcl-2 family, that plays a key role in regulating the Outer Mitochondrial Membrane (OMM) integrity and programmed cell death. Bcl-2 family proteins functionally can be classified as antiapoptotic and proapoptotic proteins. Antiapoptotic group of proteins contain four Bcl-2 Homology Domains (BH1–4) which is incorporated within OMM, and may also be in the cytosol or endoplasmic reticulum membrane. Bcl-2 related gene A1 (A1), Bcl-2, Bcl-2-related gene, long isoform (Bcl-2-xL), bcl-w and Myeloid Cell Leukaemia 1 (MCL-1) are the major members of the antiapoptotic Bcl-2 family that preserves OMM integrity by preventing the action of proapoptotic Bcl-2 proteins. Depending upon regulation and interaction of these proteins the fate of cell will be decided. Recent observations of Bcl-2 family have led to study the functions of cellular pathways [9].
Histopathological analysis of OSCC predicts the prognosis. Few histologic factors have been investigated in the literature for same either solely or as a part of multiparametric assessment and there was no significant difference of tumour size, lymphnode metastasis between the study groups and Bcl-2 expression. Similarly Camisasca DR et al., Zhang M et al., Xie X et al., Vicente JC et al., 2009 were of same opinion [10,13-15].
Lymphocytic infiltrate is a parameter used by Brandwein-Gensler M et al., which is considered as a self-determining prognostic factor in predicting survival rate. In the present study on assessing lymphocytic infiltration there was no significance between the metastatic and non metastatic Group (p=0.24). Similarly observations made by Kane SV et al., showed that LI did not predict the lymph node metastasis [11,16]. In contrast to our results Jing J et al., observed an association between LI and lymphnode metastasis, and concluded that metastasis was indirectly proportional to the lymphocytic infiltrate [17]. Brandwein-Gensler M et al., assessed LI, POI and perineural invasion to be a predictive parameter for local recurrence and survival rate. These studies thereby, suggest that lymphocytic infiltrate alone is not sensitive enough to predict metastasis. In oral cavity, lymphocytic infiltrate is associated with a protective immune response, rather than a destructive one [10].
In the present study out of 30 cases 9 cases (30%) showed POI 4 in metastatic tumour out of that 5 (16.6%) cases were well differentiated and 4 (13.4%) cases were moderately differentiated SCC. In non-metastatic group 5 (16.6%) cases showed POI 4, 4 out 5 cases were well differentiated and 1 (3%) case was moderately differentiated SCC and did not show significant results. POI 5 was observed in 2 cases of metastatic and 1 case of non-metastatic group. Whereas, Camisasca DR et al., and Brandwein-Gensler M et al., observed POI 4 and POI 5 in poorly differentiated carcinomas and was associated with local recurrence and poor prognosis [10,11].
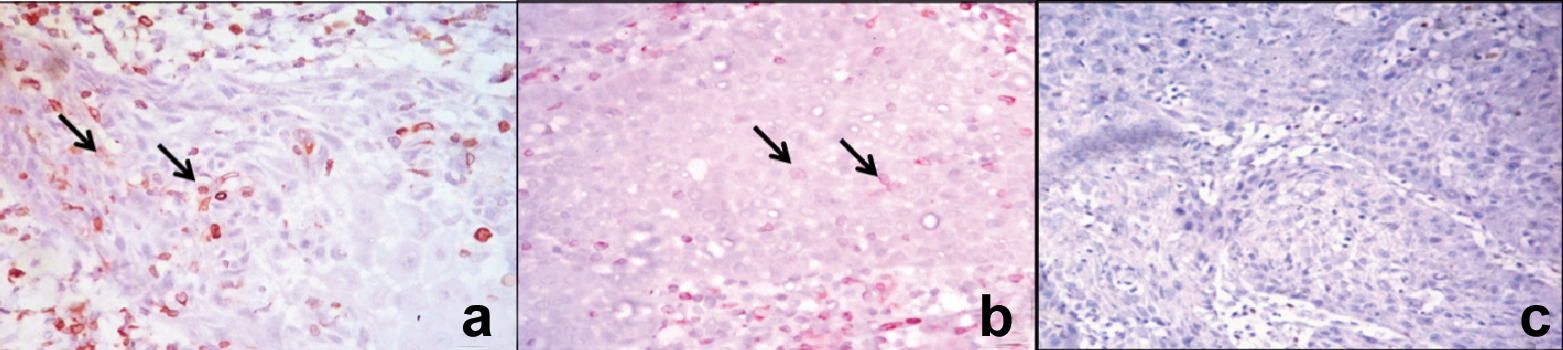
In the present study, the positive control taken was lymphnode which showed a strong positive expression [Table/Fig-3a] of the lymphocytes. Negative control tissue was stained omitting he primary antibody, showed a negative expression [Table/Fig-3b].
Photomicrographs of Bcl-2 staining showing: a) Positive control of lymphnode tissue with strongly stained lymphocytes (10X); b) Negative control (10X); c) Normal mucosa with strong Bcl-2 expression in basal layers (10X).
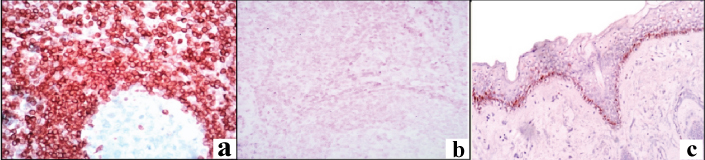
The overlying normal oral mucosa showed Bcl-2 positivity only in basal cell layer and possibly might be involved in reservoir proliferating stem cells [Table/Fig-3c]. Few ductal lining cells also showed a positive expression suggesting that these cells are also undifferentiated reservoir [11]. The results of the present study showed expression of Bcl-2 in 33.3% of OSCC among the study groups, where metastatic group showed a positive expression of 13.3% [Table/Fig-4a-c] and 20% in non-metastatic OSCC [Table/Fig-5a-c] which did not show statistically significant difference between study groups. Lymphocytic infiltrate within the tumour tissue showed a strong positive expression which was considered as internal control. Literature review revealed that in OSCC, Bcl-2 expression ranged from 7% to 60% [18]. Numerous studied showed a varying range of Bcl-2 expression from 25% to 87% (Arya et al., Suri et al., Zhang M et al., Singh BB et al., Ravi D et al.,) [2,12,13,19,20].
Photomicrographs of Bcl2 expression in metastatic OSCC: a) Moderate expression within island (400X); b) Individual cells showing strong positivity along with less distribution (400X); c) Negative expression (400X).
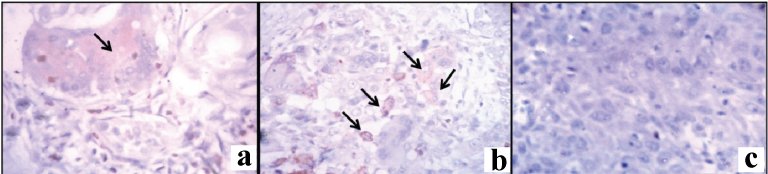
Photomicrographs of Bcl2 expression in non-metastatic OSCC: a) Moderate expression within island along with strong expression of internal control OSCC (400X); b) weakly expressed cells within the island OSCC (400X); c)Negative expression OSCC (400X).
In all these above mentioned studies, Poorly Differentiated Carcinoma (PDSCC) showed a strong positive staining, thus specifying the role of Bcl-2 in the differentiation of malignant tumour keratinocytes [1]. Due to lack of PDSCC cases, present study did not include PDSCC. The discrepancies might be attributed to varying demographic data and aetiological factors, longevity of disease and alteration in the genetic events between the different populations [1,2,10]. In our cases, the common aetiologic factors were tobacco and betel quid chewing. Though there was no significant difference between the study groups and less percentage of positive cases, there was a variation in Bcl-2 expression.
Bcl-2 over expression has also been observed in other tumour types, such as Oesophagus, breast, colon, lung, ovary and prostate carcinomas. Sudha VM et al., Singh BB et al., observed over-expression of Bcl-2 in potentially malignant disorders of oral cavity suggesting that the Bcl-2 may be associated with early stages of carcinogenesis [1,19]. This indicates potentially malignant disorders might show significant role of Bcl-2.
Induction of apoptosis by proteins of the Bcl-2 gene family is very complex and the influence of individual proteins may vary between tumours. Literature review suggests that the interaction between the Bcl-2 family proteins regulates the apoptosis either by proapoptosis or by halting the apoptosis [14]. Few studies suggest that Bcl-2-expression is high in poorly differentiated carcinoma.
Limitation
The limitations of the study are lack of poorly differentiated carcinoma cases and less sample size. On thorough literature search, there are limited studies that compared the metastatic and non-metastatic OSCC. More studies with adequate samples are required to analyse the prognosis of metastatic and non-metastatic OSCC.
Conclusion
The varying expression of Bcl-2 in this study indicates that the Bcl-2 gene product might play a role in the process of carcinogenesis. But the role of Bcl-2 in metastasis is not clearly understood, as the proteins of the Bcl-2 gene family interact with each other as homodimers and heterodimers, in the process of apoptosis. Hence, selection of molecular marker is a very important step as it provides better insight into the potential ability of a tumour and plays a vital role in the advancement of cancer progression and therapies. So, prospective studies with Bcl-2 family and more patients with follow up may provide a better understanding of these genes in progression and survival in OSCC.