Introduction
Recent times have revealed an increase in incidence of Oral Squamous Cell Carcinoma (OSCC) in young adults including those who lack association with typical risk factors such as tobacco. There are reported variations in clinical behaviour of tumours in young and older individuals.
Aim
Present study evaluated differences in clinicopathological characteristics between two groups of OSCC, below and above 40 years of age.
Materials and Methods
An analytical study was performed on two groups of OSCC patients, below and above 40 years of age. Clinicopathological parameters of site distribution, type of habit, histological grade, nodal metastasis, margin status, mitotic index and Argyrophilic Nucleolar Organizing Regions (AgNOR) count were compared. Chi-square test and Students t- test were applied for statistical analysis.
Results
Present study revealed that mean AgNOR count was significantly higher in older group (6.38) than younger group (4.27). However, no significant differences were noted in site distribution, tobacco habit, histological grade, mitotic index, nodal metastasis and status of resected surgical margins between the two age groups. A trend for increased metastasis and poor histological differentiation was also observed in the older and younger age group respectively. Most common site was buccal mucosa followed by tongue in both groups.
Conclusion
Reasons for documented variability in tumour characteristics between young and older patients are currently unclear. Difference in AgNOR count found in present study is suggestive of variability in proliferative and ploidy characteristics between different age groups and supports the hypothesis of genetic and epigenetic influences in development of oral cancer.
Introduction
OSCC was until now, chiefly considered to be a disease affecting older individuals, with usage of tobacco being a major causative factor. However, there seems to be a change in the demographic trend, with OSCCs increasingly seen in younger individuals. This has led to increasing prevalence of ‘early-onset Squamous Cell Carcinoma (SCC) which may be arbitrarily defined as SCC occurring in individuals younger than 40 years of age [1]. It is observed that there may be certain differences in the biological behaviour of tumours in younger adults. However, there is no known or proven explanation yet, attributable for these differences. In younger adults, OSCCs are sometimes seen to lack the typical association with tobacco and/or alcohol habit in addition to differences in the type and duration of habit. This raises the possibility of association of other etiological or risk factors such as viral infection and genetic susceptibility [1,2]. Differences in clinical behaviour are also observed in terms of recurrence, tendency for metastasis and survival rate. For the present analytical study, the null hypothesis was that there is no difference in clinicopathological characteristics of OSCC between individuals below and above 40 years of age. We compared two groups of individuals with OSCC categorized according to age as, below 40 and above 40 years. The objective was to assess differences in type of habit, histological features (grade of differentiation, mitotic index, and AgNOR count) and prognostic factors (lymph node metastasis and involvement of resected margins) between the two groups.
Materials and Methods
An analytical study was performed on archival formalin-fixed, paraffin- embedded tissue specimens of cases histologically diagnosed as OSCC at the Department of Oral Pathology, Manipal College of Dental Sciences, Mangalore, Manipal University, Karnataka, India. The samples were selected by convenience sampling. Inclusion criteria were histopathological diagnosis of OSCC and availability of data (age and site of tumour). Exclusion criteria were lack of data (age and site of tumour) and insufficient archival tissue. The sample comprised of 21 cases below 40 years of age and 19 cases above 40 years of age.The study was performed between July 2014 to December 2014 after approval from the Institutional Ethics Committee.
For histopathological assessment, two sections each of 4 µ thickness were obtained for each case. One section was stained with Haematoxylin and Eosin (H&E) and the other, using silver staining method for assessing AgNORs [3]. Medical records were reviewed for: histologically-proven tumour metastasis to lymph nodes, status of surgically resected margins, predominant type of tobacco habit (smoking or chewing forms). All cases were histologically categorized as well, moderately or poorly differentiated according to Bryne M et al., grading at invasive tumour front [4]. Proliferative activity in each case was assessed by two methods: AgNORs count and mitotic index. Silver staining of tissue sections was performed using a modification of the technique suggested by Kahn MA et al., using freshly prepared colloidal silver nitrate solution (50% aq. silver nitrate and gelatine in 1% aq. formic acid) and incubating the same at 500C for 45 minutes under dark conditions [3]. For each case, 50 nuclei at the invasive tumour front were assessed at 400x magnification. Each discrete dot was counted as one AgNOR and mean AgNOR count was thus calculated for each case [Table/Fig-1]. The mitotic index was determined as number of mitotic figures in ten consecutive High Power Fields (HPF) in H&E stained sections [Table/Fig-2].
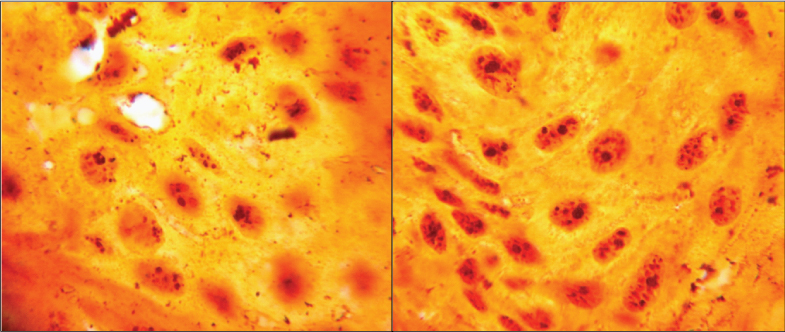
AgNORs visualised as black dots within the OSCC tumour cells (Silver stain for AgNORs, ×400) in a) <40 years and b) >40 years.
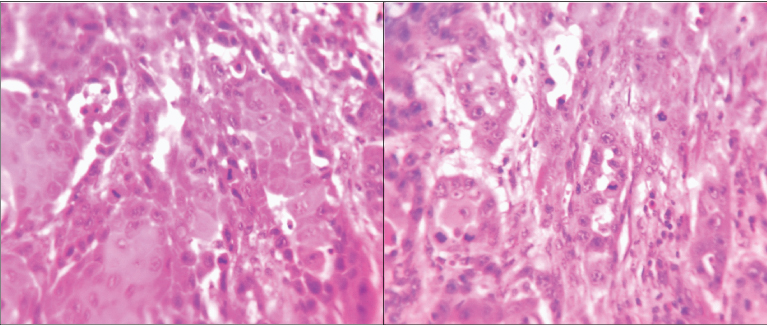
Number of mitoses assessed in 10 high power fields to calculate mitotic index (H and E stain, ×400) in OSCC in a) <40 years and b) >40 years of age.
Statistical Analysis
Descriptive statistics were used to summarize the data. Qualitative variables (type of habit, grade of differentiation, lymph node status and margin status) were assessed between the two age groups using Chi-square test; and quantitative variables (mitotic index and AgNOR count) using Independent samples t-test. In addition, Pearson’s correlation was assessed between AgNOR count and mitotic index. The level of significance was fixed at 5% and the power of the study at 80%. The data was analysed using Statistical Package for Social Sciences version 16.0.
Results
The common sites for OSCC in both age groups were buccal mucosa and tongue. Both categories of age showed a higher prevalence of males and a predominance of chewing type of tobacco habit. [Table/Fig-3] denotes comparison of clinicopathological characteristics of OSCC in young and old groups. The null hypothesis was rejected as there was a significant difference in mean AgNOR count between the two age groups. Mean AgNOR count was found to be significantly higher in older patients (mean±SD= 6.38±1.41), in comparison to the younger group (mean±SD = 4.27±0.96) (t-test, p<0.001). Pearson’s correlation analysis did not show correlation between AgNOR count and mitotic activity index (Pearson’s Rho= 0.162). Though not significant, a higher number of cases with lymph node involvement were noted among younger individuals (27.3%) as compared to older group (6%).
Tumour characteristics in ‘young’ and ‘old’ age groups in present study.
| Tumour characteristics | Number (%) of people in young age group (< 40 years) | Number (%) of people in older age group (>40years) | Test value | p-value |
|---|
| Type of habit‡Predominantly smokingPredominantly chewing | 4 (19)9 (43)NA† 8(38) | 3 (15.8)8 (42.1)NA† 8(42.1) | χ2=0.035 | 1.00 |
| Grade of differentiation‡WellModeratePoor | 1 (4.7)13 (62)7 (33.3) | 3 (15.8)12 (63.2)4 (21) | χ2=1.690 | 0.508 |
| Gender‡MaleFemale | 18 (85.7)3 (14.3) | 14 (73.7)5 (26.3) | χ2=0.902 | 0.442 |
| Lymph node involvement‡FreeInvolved | 8 (38.1)3 (14.3)NA† 10(47.6) | 15 (79)1 (5.3)NA† 3(15.7) | χ2=2.283 | 0.273 |
| Margin involvement‡Freeinvolved | 5(23.8)6(28.6)NA† 10(47.6) | 8 (42.1)8 (42.1)NA† 3(15.8) | χ2=0.054 | 1.0 |
| AgNOR count§ | Mean, SD= 4.27,0.96 | Mean, SD= 6.38,1.41 | t= -5.437 | 0.0001 |
| Mitotic index§ | Mean±, SD 2.29, 2.17 | Mean±, SD 2.63,1.89 | t= -0.535 | 0.59 |
Data not available
Chi-square test
T-test
Discussion
OSCC is generally known to be a disease predominantly affecting older males having a history of tobacco use and alcohol intake. In recent years, an increasing number of cases of OSCC in younger individuals have been documented, with studies in India paralleling the international trend [5-11]. The incidence of OSCC in patients younger than 40 years of age has been reported in various studies to range from 1% to 6% [2]. A similar trend has been observed in our institution, with the percentage of OSCC cases below the age of 40 years increasing from 7.7% between 1990-2000 to 11.7% in the time period between 2001-2013, with the youngest case reported being that of a 24 year old male. While the categorisation of ‘young’ patients being less than 40 years of age is somewhat arbitrary, it can be justified for the purpose of comparison with older individuals since the incidence of OSCC is reported to be much higher in individuals >40 years of age [2,5,8,12].
A literature search revealed that there are conflicting reports regarding differences in tumour characteristics in the aforementioned age groups [Table/Fig-4]. Some authors found tongue to be the most frequent site in younger cases [5,6,8,11,13-15]. In our study, the buccal mucosa was the most common site in both age groups, followed by tongue, mirroring findings of two other studies on Indian population [10,16]. Interestingly, different studies have observed that OSCC in younger individuals seems to be associated with fewer etiological factors such as smoking, chewing tobacco and alcohol consumption and lacks typical risk factors [1,2,5,8,12,14,17-21]. However, these factors need to be explored in the Indian context. Agrawal KH and Rajderkar SS found that 41.7% of their Indian study subjects did not consume tobacco in any form [11]. Iype EM et al also reported a similar trend, where 73% of cases below age of 31 years did not consume tobacco [22]. In addition, duration of consumption of tobacco and/or alcohol in younger individuals is considerably lesser than that in older persons, leading to scepticism as to whether these factors alone, are capable of inducing carcinogenesis [2,19,23]. This raises the question of other possible etiologic and risk factors, and leaves room for potential contribution of genetic susceptibility, immunodeficiency or viral infection in oral carcinogenesis [1,5,14,24,25]. Various studies support the role of genetic factors in carcinogenesis. [Table/Fig-4] enlists genetic differences found by various authors between OSCC in young and older individuals [24,16-31]. It has also been suggested that presence of both genetic susceptibility of individuals along with exposure to carcinogens may be responsible for increased risk of cancer [32].
There is no consensus yet regarding the clinical course and prognosis in younger individuals [33]. There are various factors that influence the outcome of OSCC. [Table/Fig-4] presents a brief summarization of clinicopathological observations of OSCC by various authors. Reports of better prognosis in younger patients could be attributed to increased awareness, detection of tumour at an earlier stage and better palliative care [2,12,18]. On the other hand, increased co-morbidities, late detection of malignancy, higher grade of tumour, genetic predisposition and increased tendency to metastasize and recur are factors that contribute to worsened prognosis in others [1,34]. In the present study, we compared lymph node metastasis and involvement of surgical resection margins by tumour between the two age groups. These are histologically assessable prognostic determinants [13,35]. We did not find any substantial difference in surgical margin status between the two age groups. However, though not statistically significant, an increasing trend for lymph node metastasis was seen in younger individuals, with a similar finding reported by some authors [17,18,23]. On the other hand, some investigators did not find any difference in prognosis of OSCC in younger individuals [7,8,13,14,21,22,33]. The possibility of distinct clinicopathological profile of OSCC in young adults warrants future research.
Observations of various studies on comparison of differences in tumour characteristics between young and older patients of OSCC.
| Parameter | Studies | Findings | Inferences |
|---|
| Common site | Muller S et al., [5]O’Regan EM et al., [6]Martin-Granzio R et al., [8]Agrawal KH and Rajderkar SS [11]Komolmalai N et al., [12]Manuel S et al., [13]Bodner L et al., [14]Fan Y et al., [15]Sun Q et al., [21] | Tongue, in younger individuals | Present study also showed highest frequency in buccal mucosa followed by tongue |
| Ramachandra NB [9]Kiran G et al., [10]Taranikanti M and Das B [16]Present study | Buccal mucosa |
| Grade of differentiation | Manuel S et al., [13]Sasaki T et al., [33] | Predominantly well-differentiated tumours in younger patients | |
| Udeabor SE et al.,[2] | Moderately differentiated tumours in less than 40 years age group | Present study showed higher tendency of poorly differentiated tumour in younger individuals (not statistically significant) |
| Prognosis - survival | Bragelmann J et al., [1] Sarkaria JN and Harari DM [34] | Young have reduced overall survival; poor prognosis | |
| Udeabor SE et al., [2]Komolmalai N et al., [12]Lacy PD et al., [18] | Younger patients have better overall survival; better prognosis | |
| Myers JN et al., [7]Martin-Granzio R et al., [8]Manuel S et al., [13]Bodner L et al., [14]Sun Q et al., [21]lype EM et al., [22]Sasaki T et al., [33] | No difference in prognosis between young and old | Conflicting reports of difference in prognosis in young and old age groups |
| Prognosis - lymph node metastasis | Sun Q et al., [21]Siriwardena BS et al., [36] | More in older patients | Present study showed a trend for higher incidence of metastasis in older age group |
| Kuriakose M et al., [17]Lacy PD et al., [18]Hilly O et al., [23] | Higher in younger individuals |
| O’Regan EM et al., [6] | Similar in both age groups |
| Proliferative activity - Mitotic index, PCNA | Siriwardena BS et al., [36] | Proliferative activity (PCNA index and number of mitoses) was higher in the older group whereas, younger persons showed a significantly greater number of nuclear aberrations histologically | Though higher number of nuclear aberrations are seen in young, OSCC in older is more proliferative |
| Present study | No significant difference in mitotic activity index in young and old | Larger sample needed to validate |
| AgNORs | Present study | Significantly higher in the older age group (p<0.001) | AgNOR may be representative of nuclear aberrations and ploidy variations as stated by some authors [37,41,42] |
| Genetic differences | Schantz SP et al., [24]: Chromosomal damage | Young population showed mutagen-induced chromosomal damage. (Bleomycin-induced chromosome breaks per cell) chromosome sensitivity was pronounced in non-tobacco users and in patients below 30 years of age. | Genetically controlled sensitivity to environmental carcinogens may be part of etiology. |
| Majchrazk C et al., [25] | OSCC in younger patients exhibits a different genotype | |
| Santos-Silva AR et al., [26]: Ploidy status of cells | Majority of cases in the younger group exhibited aneuploidy | |
| Lingen MW et al., [27]:P53 expression and mutation | 81% of cases <40 years of age overexpressed p53 but did not show mutations in exons 5-9 of the p53 gene, which are known to exist in at least 50% of older cases. | Difference in genetic mutations in young and old |
| Gawecki W et al., [28]Kostrzewska-Poczekaj M et al., [29]:Genotype | Younger individuals had higher co-occurence of risk genotypes (GSTM1(-) and Nat 2*4/6A) and reduced occurrence of XPD genotype which is responsible for DNA repair. Increased chromosomal fragility in young | Difference in the alleles involved in the metabolism of carcinogens in young and older individuals |
| Sorenson DM et al., [30] | P53 mutation was less common in young without history of alcohol and tobacco use | Molecular mechanisms behind OSCC without substance use are still unknown |
| Hafkamp HC et al., [31] | HPV more commonly detected in young head and neck cancer patients.This was related to pRb downregulation, overexpressed p16, wild type p53 expression. | Possible viral etiopathogenesis |
Tumour proliferation characteristics portray a picture of disease aggressiveness. To determine possible differences in proliferative capacity of tumours in younger patients, we histologically evaluated two surrogate markers, mitotic activity index [Table/Fig-2] and AgNORs [Table/Fig-1]. Higher number of mitotic figures is indicative of greater proliferative activity. In the present study, mean mitotic index was marginally higher in the older age group (2.29 and 2.63 in young and old patients respectively). A similar study by Siriwardena BS et al., also indicated a higher proliferative index (PCNA and mitosis) in older individuals [36]. AgNORs are loops of DNA occurring within nucleoli, in acrocentric chromosomes (chr.13, 14, 15, 21, 22) that encode for ribosomal RNA and are involved in ribosomal and protein synthesis. They are associated with non-histone proteins (NOR-associated proteins) which are argyrophilic in nature and thus, can be demonstrated as black dots by silver staining [3,27-40].On comparison between the two age groups in the present study, we found a significantly higher number of AgNORs in older adults (p<0.001). To the best of our knowledge, difference in AgNORs count between young and old patients of OSCC has not been compared previously.
AgNORs and mitotic activity are generally considered to be markers for proliferation. While both parameters were observed to be lower in younger adults in the present study, the AgNOR count and mitotic index did not statistically correlate with each other. Proliferating cells exhibit increased biosynthesis, resulting in increased rRNA, increased nucleolar activity and hence, higher number of AgNOR dots [22,41]. Keeping in view that majority of the evidence suggests that AgNORs are indicative of proliferation, the lower AgNOR count in younger individuals in the present study suggests that tumours have a less proliferative phenotype in younger individuals. On the other hand, a review of AgNORs by Underwood JC suggests that they are more representative of ploidy rather than proliferative nature, as there are differences in their presentation and assessment, besides the fact that AgNORs are found only on five chromosomes [42]. The number of AgNORs depends upon:
NOR-bearing chromosomes in the karyotype: Presence of higher number of AgNORs in older age group suggests that acrocentric chromosomes bearing NORs may be undergoing mutation in such persons;
Level of transcriptional activity, and
Stage of cell cycle: Nucleolus disperses immediately before division, causing dispersion and a relatively higher number of visible AgNOR dots [42].
However, it has also been suggested that increased number of AgNOR dots could also be due to gene amplification or chromosomal segregation [37,41]. These conflicting interpretations of AgNORs may explain the lack of correlation between AgNOR count and mitotic index. Higher AgNOR counts along with higher incidence of metastasis in older adults could indicate a difference in the genetic makeup of OSCC of older age group. Various studies have revealed differences in genotype of OSCC between young and old adults. In the future, treatment decisions and specific targeted therapy can be guided by characterising individual molecular profiles of tumour [20].
Limitation
Due to the limited sampling frame and sample size, the findings cannot be generalized to the entire population. Further large scale research is warranted to explore possible differences in OSCC between young and older individuals.
Conclusion
Many controversies surround OSCC in young adults. The present comparative study on tumour characteristics in young and older patients with OSCC did not show any significant difference in type of habit, site, grade of differentiation, lymph node status, margin status and mitotic index. A significantly higher number of AgNORs was observed in the older group. There is still ambiguity regarding the possibility of OSCC in young adults being a distinct clinicopathological entity. Large scale studies will help to shed light of differences in tumour characteristics between young and older adults. Better understanding of these differences will also potentially enable judicious employment of customised treatment modalities.
†Data not available
‡Chi-square test
§T-test
[1]. Brägelmann J, Dagogo-Jack I, El Dinali M, Stricker T, Brown CD, Zuo Z, Oral cavity tumours in younger patients show a poor prognosis and do not contain viral RNAOral Oncol 2013 49:525-33. [Google Scholar]
[2]. Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM, Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysisHead Neck Oncol 2012 4:28-34. [Google Scholar]
[3]. Kahn MA, Mincer HH, Dockter ME, Hermann-Petrin IM, Comparing flow cytometric analysis and nucleolar organizer region enumeration in archival oral premalignant lesionsJ Oral Pathol Med 1993 22:257-62. [Google Scholar]
[4]. Bryne M, Koppang HS, Lilleng R, Kjerheim A, Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic valueJ Pathol 1992 166:375-81. [Google Scholar]
[5]. Muller S, Pan Y, Li R, Chi AC, Changing trends in oral squamous cell carcinoma with particular reference to young patients 1971–2006The Emory University experience. Head Neck Pathol 2008 2:60-06. [Google Scholar]
[6]. O’Regan EM, Timon C, Sheils O, Codd M, O’Leary JJ, Toner M, Squamous cell carcinoma of the head and neck in young Irish adultsBr J Oral Maxillofac Surg 2006 44:203-06. [Google Scholar]
[7]. Myers JN, Elkins T, Roberts D, Byers RM, Squamous cell carcinoma of the tongue in young adults: Increasing incidence and factors that predict treatment outcomesOtolaryngol Head Neck Surg 2000 122:44-51. [Google Scholar]
[8]. Martin-Granzio R, Rodriguez-Campo F, Naval L, Francisco JD, Squamous cell carcinoma of the oral cavity in patients younger than 40 yearsOtolaryngol Head Neck Surg 1997 117(3):268-75. [Google Scholar]
[9]. Ramachandra NB, The hierarchy of oral cancer in IndiaInternational Journal of Head and Neck Surgery 2012 3(3):143-46. [Google Scholar]
[10]. Kiran G, Shyam NDVN, Rao J, Krishna A, Reddy BS, Prasad N, Demographics and histological patterns of oral squamous cell carcinoma at a tertiary level referral hospital in Hyderabad, India: A 5-year retrospective studyJournal of Orofacial Research 2012 2(4):198-201. [Google Scholar]
[11]. Agrawal KH, Rajderkar SS, Clinico-epidemiological profile of oral cancer: A hospital based studyIndian Journal of Community Health 2012 24(2):80-85. [Google Scholar]
[12]. Komolmalai N, Chuachamsai S, Tantiwipawin S, Dejsuvan S, Buhngamongkol P, Wongvised C, Ten year analysis of oral cancer focusing on young people in northern ThailandJ Oral Sci 2015 57(4):327-34. [Google Scholar]
[13]. Manuel S, Raghavan SKN, Pandey M, Sebastian P, Survival in patients under 45 years with squamous cell carcinoma of the oral tongueInt J Oral Maxillofac Surg 2003 32:167-73. [Google Scholar]
[14]. Bodner L, Manor S, Frigger MD, van der Waal I, Oral squamous cell carcinoma in patients twenty years of age or younger – Review and analysis of 186 reported casesOral Oncol 2014 50:84-89. [Google Scholar]
[15]. Fan Y, Zheng L, Mao MH, Huang MW, Liu SM, Zhang J, Survival analysis of oral squamous cell carcinoma in a subgroup of young patientsAsian Pac J Cancer Prev 2014 15(20):8887-91. [Google Scholar]
[16]. Taranikanti M, Das B, Risk factor profile of oral cancer patients in North East IndiaInternational Journal of Biomedical Research 2013 4(11):615-22. [Google Scholar]
[17]. Kuriakose M, Sankaranarayanan M, Nair MK, Cherian T, Sugar AW, Scully C, Comparison of oral squamous cell carcinoma in younger and older patients in IndiaEur J Cancer B Oral Oncol 1992 28B(2):113-20. [Google Scholar]
[18]. Lacy PD, Piccirillo JF, Merritt MG, Zequeira MR, Head and neck squamous cell carcinoma: better to be youngOtolaryngol Head Neck Surg 2000 122:253-58. [Google Scholar]
[19]. Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S, An analysis of risk factors for oral cancer in young people: a case–control studyOral Oncol 2004 40:304-13. [Google Scholar]
[20]. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG, Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancersHead Neck 2010 32(4):499-503. [Google Scholar]
[21]. Sun Q, Fang Q, Guo S, A comparison of oral squamous cell carcinoma between young and old patients in a single medical centre in ChinaInt J Clin Exp Med 2015 8(8):12418-23. [Google Scholar]
[22]. Iype EM, Pandey M, Mathew A, Thomas G, Sebastian P, Nair MK, Squamous cell carcinoma of the tongue among young Indian adultsNeoplasia 2001 3:273-77. [Google Scholar]
[23]. Hilly O, Shkedy Y, Hod R, Soudry E, Mizrachi A, Hamzany Y, Carcinoma of the oral tongue in patients younger than 30 years: Comparison with patients older than 60 yearsOral Oncol 2013 49:987-90. [Google Scholar]
[24]. Schantz SP, Hsu TC, Ainslie N, Moser RP, Young adults with head and neck cancer express increased susceptibility to mutagen-induced chromosomal damageJAMA 1989 262(23):3313-15. [Google Scholar]
[25]. Majchrzak C, Szybiak B, Wegner A, Pienkowski P, Pazdrowski J, Luczewsk L, Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literatureRadiol Oncol 2014 48(1):01-10. [Google Scholar]
[26]. Santos-Silva AR, Ribeiro AC, Soubhia AM, Miyahara GI, Carlos R, Speight PM, High incidences of DNA ploidy abnormalities in tongue squamous cell carcinoma of young patients: an international collaborative studyHistopathology 2011 58:1127-35. [Google Scholar]
[27]. Lingen MW, Chang KW, McMurray SJ, Solt DB, Kies MS, Mittal BB, Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5–9Head Neck 2000 22:328-35. [Google Scholar]
[28]. Gawecki W, Kostrzewska-Poczekaj M, Gajdcka M, Milecki P, Szyfter K, Szyfter W, The role of genetic factor in etiopathogensis of squamous cell carcinoma of the head and neck in young adultsEur Arch Otorhinolaryngol 2007 264:1459-65. [Google Scholar]
[29]. Kostrzewska-Poczekaj M, Gawęcki W, Illmer J, Rydzanicz M, Gajecka M, Szyfter W, Polymorphisms of DNA repair genes and risk of squamous cell carcinoma of the head and neck in young adultsEur Arch Otorhinolaryngol 2013 270(1):271-06. [Google Scholar]
[30]. Sorensen DM, Lewark TM, Haney JL, Meyers AD, Krause G, Franklin WA, Absence of p53 mutations in squamous carcinomas of the tongue in nonsmoking and nondrinking patients younger than 40 yearsArch Otolaryngol Head Neck Surg 1997 123:503-06. [Google Scholar]
[31]. Hafkamp HC, Manni JJ, Speel EJ, Role of human papillomavirus in the development of head and neck squamous cell carcinomasActa Otolaryngol 2004 124:520-26. [Google Scholar]
[32]. Cloos J, Reid CBA, Snow GB, Braakhuis BJM, Mutagen sensitivity: enhanced risk assessment of squamous cell carcinomaEur J Cancer B Oral Oncol 1996 32B(6):367-72. [Google Scholar]
[33]. Sasaki T, Moles DR, Imai Y, Speight PM, Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of ageJ Oral Pathol Med 2005 34:129-33. [Google Scholar]
[34]. Sarkaria JN, Harari PM, Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapyHead Neck 1994 16:107-11. [Google Scholar]
[35]. Dissanayaka WL, Pitiyage G, Kumarasiri PV, Liyanage RL, Dias KD, Tilakaratne WM, Clinical and histopathologic parameters in survival of oral squamous cell carcinomaOral Surg Oral Med Oral Pathol Oral Radiol 2012 113:518-25. [Google Scholar]
[36]. Siriwardena BS, Tilakaratne A, Amaratunga EA, Udagama MN, Ogawa I, Kudo Y, Analysis of histopathological and immunohistochemical differences of oral squamous cell carcinoma in young and old patients in Sri LankaJ Oral Pathol Med 2007 36:357-62. [Google Scholar]
[37]. Chowdhry A, Deshmukh RS, Shukla D, Bablani D, Mishra S, Quantitative estimation of AgNORs in normal, dysplastic and malignant oral mucosaBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014 158(2):282-87. [Google Scholar]
[38]. Hanemann JAC, Miyazawa M, Santos Souza MS, Histologic grading and nucleolar organizer regions in oral squamous cell carcinomaJ Appl Oral Sci 2011 19(3):280-85. [Google Scholar]
[39]. Khiavi MM, Vosoughhosseini S, Halimi M, Mahmoudi SM, Yarahmadi A, Nucleolar organizer regions in oral squamous cell carcinomaJ Dent Res Dent Clin Dent Prospects 2012 6(1):17-20. [Google Scholar]
[40]. Kamath VV, Sastry KARH, Nucleolar organizer regions (NORs) in oral cavityIndian J Oral Pathol 1994 01:01-11. [Google Scholar]
[41]. Piffko J, Bankfalvi A, Ofner D, Bryne M, Rasch D, Joos U, Prognostic value of histobiological factors (malignancy grading and AgNOR content) assessed at the invasive tumour front of oral squamous cell carcinomasBr J Cancer 1997 75(10):1543-46. [Google Scholar]
[42]. Underwood JC, AgNOR measurements as indices of proliferation, ploidy and prognosisClin Mol Pathol 1995 48(5):M239-40. [Google Scholar]