Materials and Methods
A cross-sectional study was designed after prior permission from the Institute’s Ethical Committee in which subjects were enrolled from patients admitted in wards of all major disciplines including Medicine, Surgery and Paediatrics. The study was conducted between start of 2010 and end of 2014. All patients with nosocomial diarrhea (Diarrhoea after 48 hours or more after hospital admission), irrespective of their age, sex or immune status were included in the study after taking proper consent. Their clinical and laboratory profile were recorded using structured questionnaire. A single stool sample was obtained from each patient. Repeat samples from the same patients were excluded from the analysis. All the samples were subjected to ELISA for detection of toxins A and B (Premier toxins A & B; Meridian Diagnostics, Inc., Cincinnati, Ohio, USA) which has a sensitivity and specificity of 96%-98% and 94%-97% respectively [11]. A cut off OD value of 0.150 at a wavelength of 450 nm was taken for result interpretation. A diagnosis of CDAD was made in all patients with stool samples positive for toxins A and B. The clinical and laboratory profile of all the CDAD cases were further analysed.
Statistical Analysis
The frequency of CDAD in patients with nosocomial diarrhoea and the epidemiological and clinical features of patients diagnosed with CDAD were expressed as percentage. The 95% confidence intervals for the frequency of CDAD among patients with nosocomial diarrhoea were calculated. The data was analysed with Stata version 12.1
Results
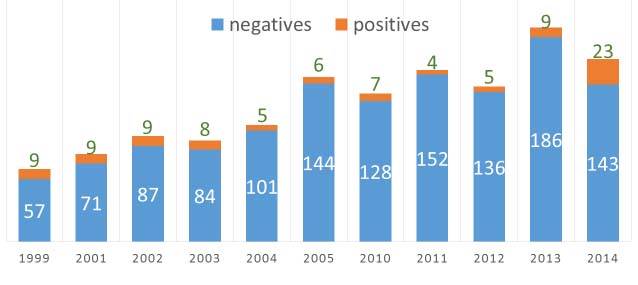
A total of 791 patients with nosocomial diarrhea were included in this study. A total of 48 patients (6%, 95% CI: 4.4%-7.7%) were diagnosed with CDAD. The incidence of CDAD in the years 2010-2014 were as follows: 7/135 (5.2%), 4/156 (2.6%), 5/141 (3.5%), 9/193 (4.7%) and 23/166 (13.8%) respectively.
A total of 31 (64.6%) of the CDAD cases were male patients, while 17 (35.4%) were female. The majority of the CDAD cases belonged to the age group of 51-60 years (n=16, 33.3%) followed by the age groups of 21-30 years (n=9, 19%), 31-40 years (n=7, 15%), >60 years (n=4, 8%), <1 year (n=4, 8%), 1-10 years (n=3, 6%), 11-20 years (n=3, 6%) and 41-50 years (n=2, 4%).
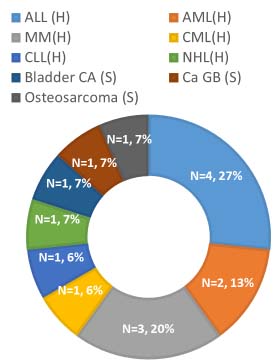
Malignancy (n=15, 31%) was the most common underlying pathological condition in cases of CDAD. Haematological malignancy (n=12, 25%) was more common than solid organ malignancy (n=3, 6%) [Table/Fig-1]. A positive history of abdominal surgery was there in eight patients (16%). A total of six patients (12%) were transplant recipients (n=5, 10%- bone marrow, n=1, 2% - liver).
Pie chart showing different haematological and solid organ malignancies in patients with Clostridium difficile associated diarrhoea.
{(H): Haematological malignancies, ALL: Acute lymphoid leukaemia, AML: Acute myeloid leukaemia, MM: Multiple Myeloma, CML: Chronic myelocytic leukaemia, CLL: Chronic lymphocytic leukaemia, NHL: Non-Hodgkin’s Lymphoma, (S): Solid organ malignancies, Ca GB: Carcinoma Gall bladder, Bladder Ca: Bladder cancer,O (S): Osteosarcoma }.
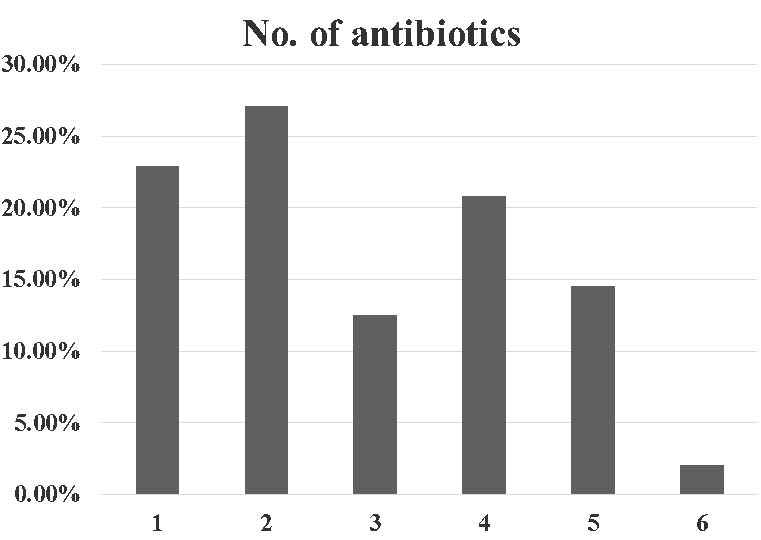
All the cases of CDAD had a history of intake of at least one dose of antibiotic. The average number of antibiotic intake per patient was calculated to be 2.8 [Table/Fig-2]. Most common antibiotic used in the patients of CDAD was third generation cephalosporins. The other common antibiotics used in patients who developed CDAD were carbapenems, aminoglycosides and fluoroquinolones [Table/Fig-3].
Bar chart showing maximum number of antibiotics received by each patient. The X axis shows maximum number of antibiotics received by a particular patient. The Y axis shows percentage of patients with Clostridium difficile associated diarrhoea receiving that number of antibiotics.
Frequency of drugs used in the Clostridium difficile associated diarrhoea patients.
| Drugs | Frequency |
|---|
| 1 | Antibiotics | 48 (100%) |
| I | Third generation cephalosporins | 27 (56.25%) |
| II | Carbapenems | 16 (33.33%) |
| III | Aminoglycosides | 13 (27.08%) |
| IV | Fluoroquinolones | 13 (27.08%) |
| V | Piperacillin- tazobactam | 10 (20.83%) |
| VI | Antifungals | 5 (10.41%) |
| VII | Colistin | 5 (10.41%) |
| VIII | Clindamycin | 4 (8.33%) |
| IX | Cotrimoxazole | 3 (6.25%) |
| X | Penicillin | 3 (6.25%) |
| XI | Ampicillin | 2 (4.16%) |
| XII | Amoxicillin | 1 (2.08%) |
| 2 | Proton pump inhibitors | 5 (10.4%) |
| 3 | Anticancer drugs | 12 (25%) |
| 4 | Immunosuppressive agents | 8 (16%) |
The trend of antibiotic usage from 2010 to 2014 showed an increase in the usage of carbapenems, clindamycin and colistin in the year 2014 [Table/Fig-4].
Trends in antibiotic use from 2010-2014.
| Year | Cases | Average number of antibiotics | 3rd generation cephalosporin | Piperacillin tazobactam | Carbapenem | Clindamycin | Colistin |
|---|
| 2010 | 7 | 3 | 5 | 1 | 2 | 0 | 0 |
| 2011 | 4 | 1.7 | 4 | 1 | 0 | 0 | 0 |
| 2012 | 5 | 3.2 | 4 | 1 | 2 | 0 | 1 |
| 2013 | 9 | 2.9 | 4 | 3 | 2 | 1 | 0 |
| 2010-2013 | 25 | 2.7 | 17 | 6 | 6 | 1 | 1 |
| 2014 | 23 | 2.9 | 10 | 4 | 10 | 3 | 4 |
History of intake of Proton Pump Inhibitors (PPI) was present in only 10.4% of patients. A total of twelve patients (25%) were receiving anti-cancer drugs. A total of eight patients (16%) were on one or more immunosuppressant.
Mean duration of hospital stay before development of nosocomial diarrhoea in CDAD cases was 9.8 days. Mean duration of diarrhoea in CDAD cases was eight days while mean frequency was around nine times per day.
Diarrhoea was commonly associated with fever and abdominal pain. The most common laboratory abnormality observed was anaemia [Table/Fig-5]. The patients were not followed up to note their clinical outcomes.
Clinical Laboratory findings in patients of Clostridium difficile associated diarrhoea.
| Clinical and laboratory parameters | No. of patients |
|---|
| Fever | 24 (50%) |
| Abdominal pain | 19 (39.6%) |
| Anaemia | 26 (54.16%) |
| Raised Alkaline phosphatase | 15 (31.25%) |
| Leucocytosis | 13 (27.08%) |
| Thrombocytopenia | 8 (16.67%) |
| Neutropenia | 6 (12.5%) |
| Raised urea | 4 (8.33%) |
| Raised creatinine | 2 (4.16%) |
Discussion
In a small study done in 1999, out of 66 patients with antibiotic associated diarrhoea, stool samples of nine patients (7.3%) were positive by toxin ELISA [4]. Haematological malignancy (5/9) was the most common comorbidity in that study also. Most common implicated antibiotics were third generation cephalosporins (66.6%) followed by aminoglycosides (55.5%). In another study by our group, done from 2001-2005, a total of 37 (7.1%) stool samples were positive for toxin ELISA (11.2%, 9.4%, 8.6%, 5% and 4% respectively from 2001-2005) [6]. The prevalence in that study was on a decreasing trend from 2001- 2005. Looking at our results from 2010-2014, the decreasing trend was continued but there was a significant spurt in 2014. Through, our studies, we initially highlighted the problem of Clostridium difficile and stressed on better antimicrobial stewardship programmes and hospital infection control policies. The numbers decreased in the ensuing years with active participation from the administration, clinicians and diagnosticians. The sudden increase in the number of cases in 2014 showed us, that we are far from our goal. This spurt could have been attributed to increased use of carbapenems, clindamycin and colistin in 2014 as compared to their cumulative usage from 2010-2013 [Table/Fig-6].
Bar chart showing number of patients with nosocomial diarrhoea and those diagnosed with Clostridium difficile associated diarrhoea in each year. X-axis showing the year in question and Y axis showing number of patients without Clostridium difficile associated diarrhoea (blue) and those diagnosed with Clostridium difficile associated diarrhoea (orange). (Includes data on file from previous years)
The increased use of higher antibiotics in our institute was to meet the needs of increasing resistance. According to the Centre for Disease Dynamics Economics and Policy, the resistance for piperacillin- tazobactam and carbapenems in Escherichia coli increased from 28% and 5% in 2010 respectively to 37% and 11% in 2014 respectively. Similarly, the resistance for piperacillin- tazobactam and carbapenems in Klebsiella pneumoniae increased from 60% and 34% respectively in 2010 to 63% and 57% respectively in 2014 [12].
Most patients enrolled in 2001-2005 study were from the haematology/oncology ward (67.5%) and were on third generation cephalosporins (50%) or quinolones (35%). Compared to both our previous studies, the number of samples sent for testing in the present study has gone up, indicating that the previous studies have been able to create awareness about the need for testing in patients with nosocomial diarrhoea [Table/Fig-6]. The lacunae in our previous study were that, we were not able to analyse the clinical and laboratory parameters of the patients who were diagnosed with CDAD. Also, the use of drugs other than antimicrobials was not noted. With this study, we have tried to answer several questions that were left unanswered by our previous studies [4-6].
We speculate an increasing trend in the number of CDAD cases, as the problem of resistance is far from being controlled, especially in resource limited settings. The rampant use of higher antibiotics continues without proper implementation of stewardship programme. Ours is an apex care institute, where we mostly deal with patients who are referred from primary, secondary and tertiary care centres. Most patients are already on antibiotics when they were referred to our institute. To target, a decline in the number of CDAD cases, there needs to be robust implementation of national programmes in the lower centres also. Also, there is a need for availability of routine diagnostic services, especially in resource limited settings. In places, where ELISAs cannot be used due to lack of infrastructure and technical expertise, Rapid Diagnostic Tests (RDT) would play a big role. The use of this point of care tests would allow for early diagnosis and treatment of suspected cases.
Infection with C. difficile ranges from asymptomatic colonization to severe disease. Around 1%-15% of individuals are normally colonized with C. difficile [13]. The frequency of colonisation increases to 20%-40% in hospitalised patients [14]. This is the reason why toxin detection and not just culture is used for the diagnosis of CDAD [15]. It was noted in our study that elderly patients were more commonly affected than any other age group. This was in concordance with other studies [16]. In an analysis, it was calculated that, above the age of 18, with every passing year, the risk of infection with C. difficile increases by 2%. This is possibly because of the worsening immune status and increasing comorbidities with age [17]. The other important age group with higher incidence of CDAD is the paediatric age group [18,19]. Around 6% of our patients were under the age of 10 years. The diagnosis of CDAD in children is challenging as asymptomatic colonization of C. difficile is even more common in early infancy [13]. Therefore, toxin demonstration is of utmost importance in children for a definite diagnosis.
Malignancy is one of the most important risk factors associated with increased C. difficile carriage [Table/Fig-7] [8-10,20]. In a study conducted in China, the carriage rate in cancer patients was significantly higher than non-cancer patients [21]. The possible reasons attributed to the increased carriage are altered gut flora and increased hospital stay in cancer patients. Transplant recipients are also vulnerable to CDAD because of similar reasons. In a recent meta-analysis, incidence of CDAD was nine times higher in haematopoietic stem cell transplant cases than among all other hospital stays [22]. Abdominal surgery has also been known as a risk factor for CDAD mostly because of the preoperative exposure to antibiotics [Table/Fig-7] [8,10,20].
Studies from other parts of India in the recent past reporting the incidence of CDAD and associated risk factors.
| Authors and study | Incidence and demography | Risk factor (other than antibiotic use) | Clinical and laboratory parameters |
|---|
| Vaishnavi C et al.,(2009-2012)Retrospective studyPunjab [8] | 17.5%All age grouptested by ELISA | Gastrointestinal diseases (21.1%), renal diseases (20.8%), surgical conditions (20.7%), hepatic disorders (18.5%) and cancers (17.6%) | Fever (41%) Abdominal pain (37.9%) |
| Justin S et al., (2012-2013)Prospective studyKarnataka [9] | 10.9%Paediatric age grouptested by ELISA | Underlying diseases (7/15)Surgery (1/15) | Fever (6/15) |
| Kaneria MV and Paul SProspective studyTamil Nadu [10] | 10%All age grouptested by ELISA | Exposure to proton pump inhibitors | Leucocytosis (40%) |
| Ingle M et al.,(2009-2010)Prospective studyMaharashtra (20) | 17%All age grouptested by ELISA | Malignancy, ICU stay, Exposure to immunosuppressive and chemotherapeutic agents | Fever (47%), Abdominal pain (41%) |
It has been long been established that antibiotics have been the most important risk factor for CDAD as they disrupt the normal gut flora and allow the multiplication of Clostridium difficile [13]. Almost all the antibiotics that are commonly used are implicated in CDAD [23]. Cephalosporins were the most common group of antibiotics used in patients who developed CDAD in our study. This finding was in concordance with several other studies [24]. A significant percentage of the patients were also on PPI. Several studies in past have linked PPI intake with occurrence of CDAD [17]. PPIs work by decreasing the gastric acid and thereby increasing the chances of survival of Clostridium difficile [25].
The diarrhoea in CDAD is commonly associated with abdominal pain. The presence of fever, leucocytosis and hypoalbuminemia in relevant settings should warn the physician about the possibility of CDAD [Table/Fig-7] [8-10,20]. In our study, although a number of patients presented with fever, abdominal pain and leucocytosis, majority of the patients with CDAD did not have any of these symptoms. Thus, there is need for heightened suspicion in patients with nosocomial diarrhoea, even in absence of suggestive symptoms.
Limitation
The study design was cross-sectional and therefore, only frequency of associated risk factors in patients of CDAD was calculated. No comparison with the patients who were negative for toxins by ELISA was done. The cases were not followed up for the mortality rate determination.
Conclusion
C. difficile appears to be an important cause of nosocomial diarrhoea in the present study area. The high positivity of CDAD cases in recent years stresses the need for the availability of routine diagnostic services for detection of C. difficile toxins. In patients with high risk of CDAD, increased suspicion would lead to early diagnosis and prompt alleviation of associated morbidity. The findings of our study re-emphasises on the rational use of antibiotics, especially in patients with longer anticipated duration of stay. The antibiotic stewardship programmes and hospital infection control initiatives should not be limited to a selected few. They should cover every one starting from the primary care physicians in the rural settings to specialists working in apex care institutes.