Combined Thrombophilia in a Young Male Presenting as Life Threatening Pulmonary Embolism
Akshyaya Pradhan1, Ayush Shukla2, Mili Jain3, Anupam Mehrotra4, Rishi Sethi5
1 Assistant Professor, Department of Cardiology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
2 Senior Resident, Department of Cardiology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
3 Assistant Professor, Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
4 Assistant Professor, Department of Cardiology, Ganesh Shankar Vidyarthi Memorial College, Kanpur, Uttar Pradesh, India.
5 Professor, Department of Cardiology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ayush Shukla, Senior Resident, Department of Cardiology, King George’s Medical University, Lucknow-226003, Uttar Pradesh, India.
E-mail: ayushgeorgian@gmail.com
Combined hereditary thrombophilia is an uncommon entity associated with higher risk of early onset thrombosis. We report a case of 39-year-old male with combined deficiency of natural anticoagulants (protein C, S and anti thrombin) along with hyper homocystenemia and factor V Leiden mutation, presenting with life threatening bilateral pulmonary embolism. The clinical implications of combined thrombophilia are also discussed.
Anti thrombin, Fibrinolysis, Protein C, Protein S
Case Report
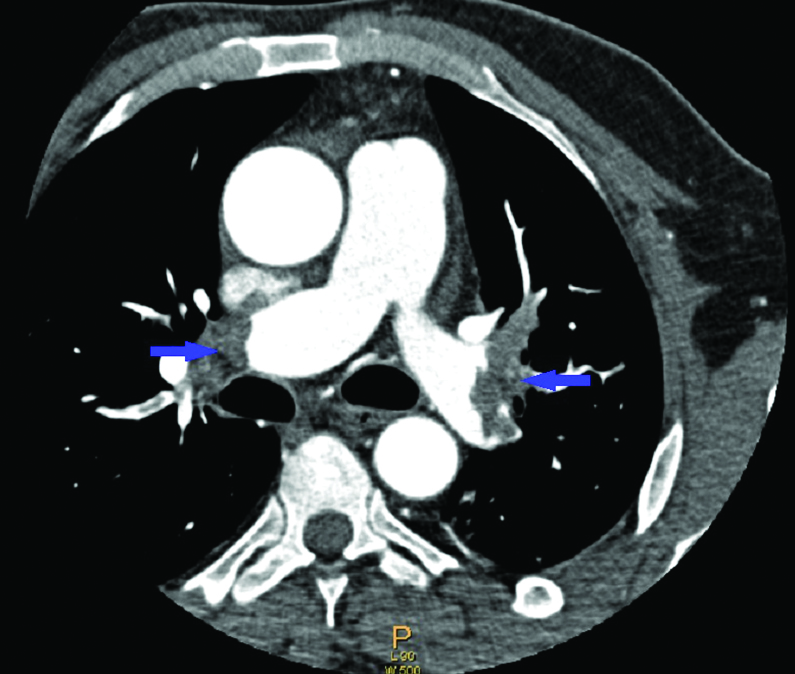
A 39-year-old male presented in the Emergency Department with complaints of chest pain, breathlessness at rest and dizziness for four days. He had no history of fever, cough, lower limb pain, swelling, liver disease, renal disease, recent surgery, immobilisation, long travel or any addiction. He had no such episode previously. His family history was unremarkable. On examination his peripheral extremities were cold and clammy and pulse rate was 110 bpm, blood pressure was 90/60 mm Hg, respiratory rate was 32/min and jugular venous pressure was raised 10 cm above sternal angle. Other systemic examination was unremarkable. SpO2 at room air was 85%. ECG revealed T wave inversion in anterior leads and S1Q3T3 pattern. On 2D echocardiography there was no left ventricular regional wall motion abnormality with normal left ventricular ejection fraction but there was severe Tricuspid Regurgitation (TR) with enlargement of both right atrium and ventricle and features of Right Ventricular (RV) dysfunction {RV fractional area change of 25 % and Tricuspid Annular Plane Systolic Excursion (TAPSE) of 13 mm}. Biochemical markers troponin T (0.12 ng/ml) and Pro- BNP (2346 pg/ml) were deranged. A possible diagnosis of Pulmonary Embolism (PE) was made on the basis of clinical examination, ECG and echocardiography. CT pulmonary angiogram revealed intraluminal-filling defects in both right and left pulmonary arteries, bilateral lobar arteries and few segmental and sub segmental arteries suggestive of thrombus [Table/Fig-1]. Doppler of bilateral lower limb showed normal flow pattern. His haemogram, serum vitamin B12, folic acid, liver function, and renal function test were within reference ranges. Patient was thrombolysed with streptokinase 2.5 lac units bolus followed by intravenous infusion of 1 lac units/hr for 72 hours. Patient improved and his breathlessness was relieved over course of 5 days. Patient was taken on an overlap of low molecular weight heparin subcutaneously and warfarin 5 mg OD till an International Normalised Ratio (INR) of 2.0-3.0 was achieved and patient was discharged on warfarin 5 mg OD. Pre discharge echocardiogram revealed normal RV functions with trace TR.
Thoracic computed tomography angiography showing bilateral thrombi in the right and left branches of the pulmonary artery.
Patient remained asymptomatic on routine follow up. On his subsequent visit three month later he was investigated for thrombophilia profile after interrupting warfarin and putting on low molecular weight heparin. His-protein C was 35% (range 89-150%), protein S level was 30% (range 91-150%), and antithrombin activity was 36% (range 79-124%). His plasma homocysteine levels were high (48.12 μmol/L). Polymerase chain reaction was positive for heterozygous factor V Leiden mutation. Antiphospholipid screening was negative. Patient was again put on warfarin and continues to remain on follow up. The final diagnosis was combined thrombophilia presenting with life threatening bilateral PE in a young male.
Discussion
Thrombophilia is the increased tendency to develop Venous Thromboembolism (VTE) due to predisposing congenital and or acquired factors. VTE affects approximately one-two people per 1000 in Western general population [1]. Massive PE accounts for 5% to 10% of cases [2]. Life-threatening pulmonary embolism sufficient to require thrombolysis is uncommon in a young healthy patient in the absence of a provocating factor. Combined thrombophilias due to presence of more than one inherited coagulation defect have been reported to constitute 7.8%-8.3% of thrombophilic patients [3,4]. Combined defect in four or more factors is seen in 1.2% of these cases and a very low proportion of 0.1% of overall thrombophilia cases. Our case was rare with multiple thrombophilic abnormalities namely deficiency of natural anticoagulants (protein C, S and anti thrombin), hyper homocystenemia and presence of factor V Leiden mutation. The patient was selected for inherited thrombophilia testing as per the thrombophilia guideline recommendations (unprovoked VTE in < 40 year). The testing for thrombophilia is recommended in selected population including: those presenting with venous thrombosis at an early age (<40) and who are from apparent VTE prone families, children with purpura fulminans, pregnant women at risk of venous thrombosis [5].
Deficiency of natural anticoagulants (protein C, S and anti thrombin) is rare (<0.5% of general population) but associated with higher risk of thrombosis (5-10 fold) with an annual VTE incidence >1% [6]. On the other hand factor V Leiden mutation and prothrombin gene mutation are commoner (5% of general population) but associated with lower risk of thrombosis (2-5 fold) with an annual VTE incidence of < 0.5% per year [7]. Combined defects have a more severe phenotype with higher thrombosis risk (20-50 fold), earlier first episode and higher recurrence [8]. Our case also presented at an early age with life threatening bilateral pulmonary embolism. Laczika K et al., similarly reported a case of combined inherited thrombophilia (type I anti thrombin deficiency and prothrombin G20210A mutation) in a 19-year-old female presenting with unilateral chronic thromboembolic pulmonary hypertension secondary to pulmonary embolism [9]. Friedline JA et al., reported combined factor V and prothrombin gene mutations in a 36-year male presenting with recurrent bilateral lower limb venous thrombosis [10]. The usefulness of thrombophilia testing in predicting recurrent VTE is still a matter of debate due to conflicting reports [11].
The initiation, intensity and duration of anticoagulant therapy however do not differ in patients with or without heritable thrombophilia [5]. The diagnostic evaluation should be delayed till management of acute thrombotic episode as was in our case. Patients on coumarin therapy should be shifted to heparin before testing to avoid interference with protein levels. The decision for duration of therapy (lifelong or not) is made regardless of result of heritable thrombophilia testing. It is taken with reference to whether or not a first episode of venous thrombosis was provoked or not, other risk factors, and risk of anticoagulant therapy-related bleeding.
Testing for case finding in asymptomatic relatives of rare combined thrombophilias is not recommended, as it is not predicted by family history. In a prospective follow up of asymptomatic relatives the risk of thrombosis was low [12]. No family screening was done in our case. There is no increased risk of death in individuals with thrombophilia, not even in those with a history of thrombosis [4]. The work up for hereditary thrombophilia should still be sought for in young patients with unprovoked venous thromboembolism so as to identify these rare disease phenotypes of combined defects. Also the documentation is important to add to the knowledge of the disease profile.
Conclusion
Thrombophilia testing should be carried out in young (<40 year) individuals with unprovoked venous thrombosis. Multiple defects may be contributory in a single individual and hence testing for all risk factors is recommended. The thrombosis testing should however be deferred at the time of acute episode due to alterations in factor levels, a complete work up should be planned once the patient is stabilized. Genetic mutations can however be studied anytime.
[1]. Spek CA, Reitsma PH, Genetic risk factors for venous thrombosisMol Genet Metab 2000 71:51-61. [Google Scholar]
[2]. Godhaber SZ, Pulmonary embolism. In: Mann DL, Zipes DP, Libby P, Bonow RO, Brawnwald E, editorsBrawnwald’s Heart Disease- A textbook of cardiovascular medicine 2015 PhiladelphiaSaunders:1664-1681. [Google Scholar]
[3]. Losonczy H, Nagy A, Toth O, Balassa K, David M, Keckes M, Prevalnece and clinical significance of single and combined inherited thrombophliasBlood 2005 106:1632 [Google Scholar]
[4]. Pabinger I, Vossen CY, Lang J, Conard J, Garcia-Dabrio MC, Miesbach W, Mortality and inherited thrombophilia: results from the European Prospective Cohort on ThrombophiliaJ Thromb Haemost 2012 10:217-22. [Google Scholar]
[5]. Baglin T, Gray E, Greaves M, Clinical guidelines for testing for heritable thrombophiliaBritish Journal of Haematology 2010 149:209-20. [Google Scholar]
[6]. Rosendaal FR, Reitsma PH, Genetics of venous thombosisJ Thromb Haemost 2009 7(Suppl 1):301-04. [Google Scholar]
[7]. Middeldorp S, Levi M, Thrombophilia: an updateSemin Thromb Haemost 2007 33:563-72. [Google Scholar]
[8]. Franchini M, Utility of thrombophilia testingClin Chem Lab Med 2014 52(4):495-97. [Google Scholar]
[9]. Laczika K, Lang IM, Quehenberger P, Manhalter C, Muhm M, Klepetko W, Unilateral chronic thromboembolic pulmonary disease associated with combined inherited thrombophiliaChest 2002 121:286-89. [Google Scholar]
[10]. Friedline J A, Ahmad E, Garcia D, Blue D, Ceniza N, Mattson JC, Combined factor V Leiden and prothrombin genotyping inpatients with thromboembolic episodesArch Pathol Lab Med 2001 125:105-11. [Google Scholar]
[11]. Kyrle PA, Rosendaal FR, Eichinger S, Risk assessment for recurrent venous thrombosisLancet 2010 376:2032-39. [Google Scholar]
[12]. Langlois NJ, Wells PS, Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic reviewThrombosis and Haemostasis 2003 90:17-26. [Google Scholar]