Primary Urethral Carcinoma (PUC) is considered as a rare tumour, accounts for less than 1% of all malignancies and an incidence rate of four cases per million patients. Incidence increases with the patient’s age and most commonly present in seventh decades. Urothelial carcinoma is the most common type (76%) of the PUC, adenocarcinoma accounts for less than 5% of the PUC. No definitive protocol for tumour management for urethral adenocarcinoma has been described in the literature due to lack of prospective study and scarcity of the cases. Treatment usually depends on the site and stage of the tumour. We hereby report a case of 33-year-old male patient with urethral adenocarcinoma of bulbomembranous urethra spread to the prostatic urethra and left side inguinal lymph node. He was treated through multimodal therapy with surgery plus adjuvant chemotherapy.
Prostate, Tumour, Urethral cancer, Urethral stricture
Case Report
A 33-year-old male patient presented to outpatient department with the complaint of protruding mass from perineal urethrostomy meatus for last one month. In addition, there was the complaint of obstructive voiding and per urethral bloody discharge of same duration, but no history of pain or haematuria. He had undergone the first stage Johanson’s urethroplasty with perineal urethrostomy six months back for the pan-anterior urethral stricture. In first stage Johanson’s urethroplasty, corpora spongiosa and anterior urethra were laid open ventrally in the stricture part. After that, the margin of the corpora spongiosa was sutured to the skin and proximal perineal urethrostomy was done. During urethroplasty, there was no suspicious lesion present, hence no biopsy was taken. The patient was planned for retubularization of the urethral plate (second stage Johanson’s urethroplasty) after six months. There was a history of obstructive voiding due to the urethral stricture for two years before urethroplasty operation.
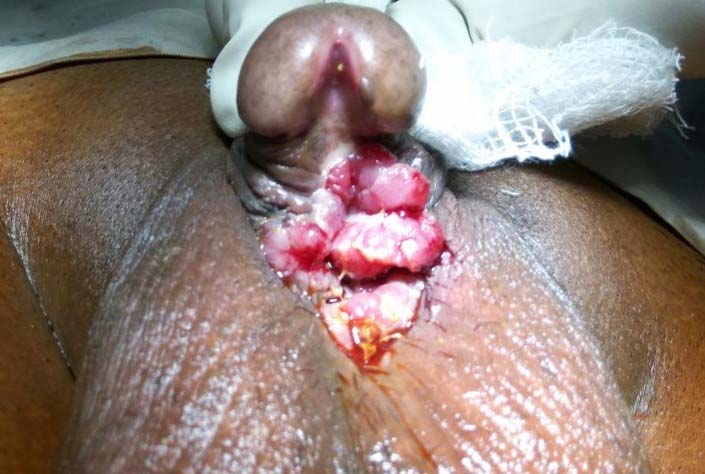
General examination was essentially normal except a 1.5 cm non-tender, palpable, mobile, hard left inguinal lymph node. On local examination, an ulceroproliferative growth (2 cm X 2.5 cm) was seen over ventral aspect of the proximal penis near neourethral meatus [Table/Fig-1]. On palpation, an underlying indurated mass (3 cm X 5 cm) with minimal mobility was present. Local tenderness was absent and minimal bleeding on touch was present. Digital rectal examination was within normal limit.
An ulceroproliferative growth seen at ventral aspect of proximal penis through the meatus of perineal urethrostomy.
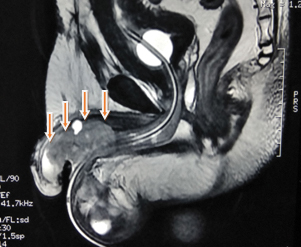
Incisional biopsy was taken from the margin to know the nature of the growth and histology report showed well-differentiated adenocarcinoma. MRI of the whole abdomen and pelvis was done for the local staging. It revealed a lobulated margined, poorly enhancing and partly necrotic, infiltrating lesion in the undersurface of the proximal half of the penis, involving corpora spongiosum and mildly adjacent corpora cavernosa. The lesion measures about 51 mm (anteroposterior) X 22 mm (mediolateral) X 24 mm (superior-inferior). The lesion was mildly hyperintense in T2 and isointense in T1 [Table/Fig-2]. Blood biochemistry test, liver function test and chest X-ray were within normal limit. Cystourethroscopy was done to see the proximal extent of the tumour. Mid and distal prostatic urethra was involved along with the bulbomembranous urethra [Table/Fig-3].
MRI of pelvic region in T2 weighted image showing a lobulated margined, mildly hyperintense, poorly enhancing and partly necrotic, infiltrating lesion (5.1 X 2.2 X 2.4 cm) in the undersurface of the proximal half of the penis, involving corpora spongiosum and mildly adjacent corpora cavernosa (marked with arrows).
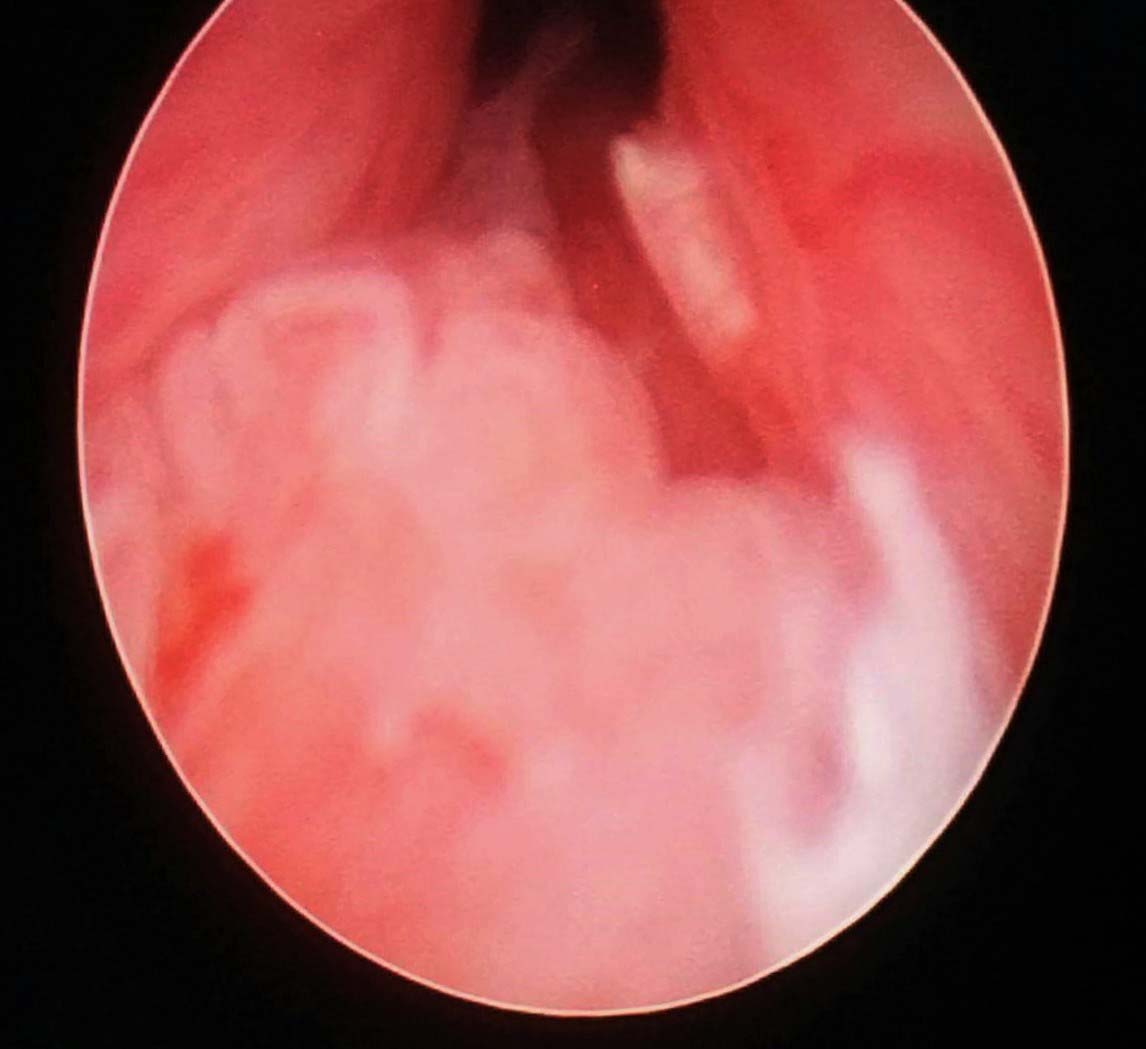
Cystourethroscopy showed that tumour extended proximally to the prostatic region. Bladder neck and bladder were not involved.
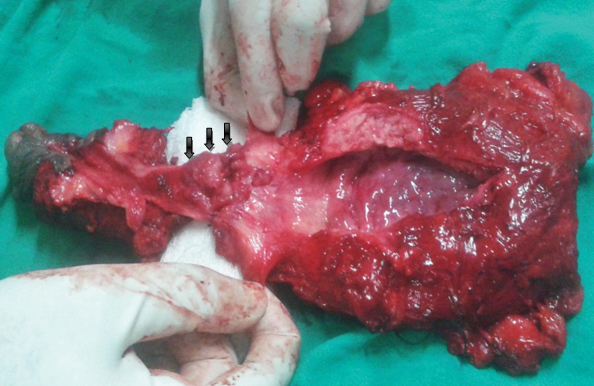
En bloc radical cystectomy, pan urethrectomy and total penectomy with ileal conduit urinary diversion were done [Table/Fig-4]. Simultaneously, bilateral Pelvic Lymph Node Dissection (PLND) was also performed. Bilateral Inguinal Lymph Node Dissection (ILND) was performed in a different sitting after two weeks. Postoperative recovery was uneventful. The histopathological report showed well-differentiated adenocarcinoma of urethra involving periurethral muscle, corpora spongiosa and distal half of prostate (T2) [Table/Fig-5]. Corpora cavernosa, proximal prostate, vas deferens, seminal vesicles and bladder were free of tumours. All the pelvic lymph nodes were reactive. Left side inguinal lymph node showed metastatic deposits (N1). Adjuvant chemotherapy with 5-Fluorouracil (5-FU) and three cycles of cisplatin was given. The patient is now on close follow up for last nine months, no recurrence has been seen yet.
En bloc excised specimen of radical cystoprostatectomy, total penectomy and urethrectomy. Tumour at bulbomembranous urethra extended proximally to prostatic urethra (marked with arrows). Bladder and bladder neck were free of tumour.
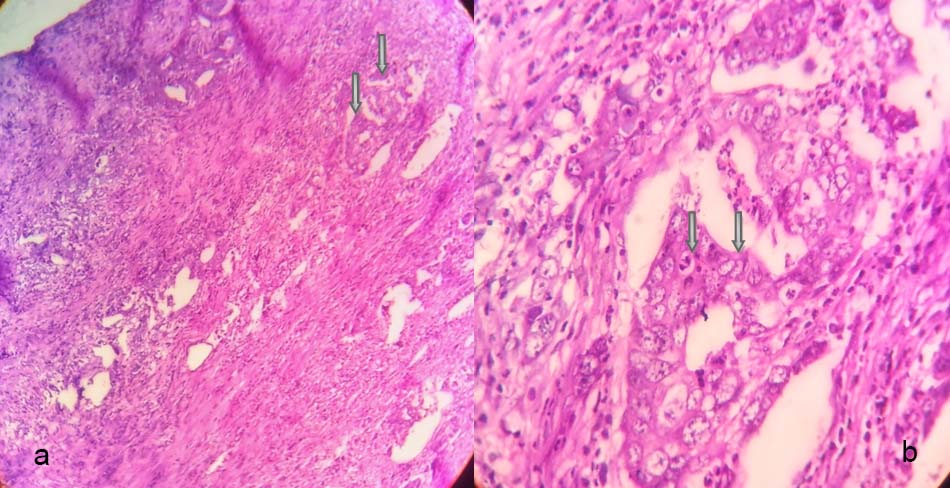
a) Low (10X) and b) High (40X) magnification images showing adenocarcinoma of the urethra with the malignant glands (marked with arrows) lined by pleomorphic cells with high nucleocytoplasmic ratio and prominent nucleoli.
Note: Haematoxylin and Eosin (H&E stain) used
Discussion
PUC is a rare type of malignancy which accounts for about less than 1% of all malignancies [1]. According to the recent analysis of Surveillance, Epidemiology and End Results (SEER) database, a male is two times more commonly affected than female and the peak age is at the eighth decade of life [2]. The incidence of Urethral Cancer (UC) is almost negligible before the fifth decade of life (0.2 cases/million) [2]. The age of index patient was only 33 years.
Although, there is no definitive causative factor identified but many aetiological factors have been postulated like chronic irritation, urethritis, urethral stricture, external beam radiation therapy/brachytherapy and human papilloma virus or other viral infections [3]. In the present case, urethral stricture and urethritis were the probable causative factors.
UC can be categorized according to location and histopathology. SEER database based study revealed that among 2065 patients, urothelial carcinoma was the most common subtype of UC (77.6%), adenocarcinoma accounts for only 5% of cases [4]. Bulbomembranous urethra (60%) was the most common site of UC followed by penile urethra (30%) and prostatic urethra (10%) [5]. The index patient had adenocarcinoma of the bulbomembranous urethra with extension to the distal prostatic urethra.
At the time of presentation, 45%-57% of the patients of PUC have symptoms of advanced disease [6]. Symptoms include visible haematuria or bloody urethral discharge (62%), extraurethral mass (52%), obstructive voiding (48%), pelvic pain (33%) or urethrocutaneous fistula (10%) [6]. Over the time, the tumour invades the vascular spaces of corpus spongiosum, periurethral muscle, corpus cavernosa, perineum, proximal/distal urethra, bladder and surrounding structures or it can metastasize through lymphatic embolization to regional lymph nodes or rarely metastasize to distant organs [5].
Anterior urethral tumour primarily spread to superficial and deep inguinal lymph nodes. In contrast, posterior urethral tumours drain into external, obturator and an internal iliac group of lymph nodes. Palpable inguinal lymph nodes are present in 20% of the patients with UC and almost always positive for the malignancy [5]. Haematogenous metastasis is rare; lung, liver, bone and brain are the sites in decreasing order of frequency [7]. Our patient was presented with per urethral protruding mass and obstructive voiding with palpable left inguinal lymph node without distant metastasis (T2N1M0).
Males with UC should be evaluated by detailed history, cystourethroscopy, examination under anaesthesia, radiological imaging like CT/MRI of pelvis and abdomen and CT/X-ray chest [3]. MRI provides best anatomical details of tumour extensions and lymph nodes involvement. In MRI, urethral lesions appear hypointense on T1 weighted image and hyperintense on T2 weighted image [8]. Histopathological Examination (HPE) is necessary for definitive diagnosis.
There is no definite protocol for treatment and follow up of UC due to the scarcity of cases and heterogenicity of the disease. Treatment is individualized and depends on the location and stage of the tumour [5]. Surgical excision of the tumour is the mainstay of treatment for PUC. Superficial, small tumour of the anterior urethra can be treated with transurethral resection and local excision or distal urethrectomy or perineal urethrostomy [5]. But advanced and proximal urethral tumours require more extensive surgery like partial/total penectomy, en bloc radical cystoprostatectomy, urethrectomy, total penectomy with or without pubectomy and bilateral PLND +/- ILND [5]. There are no survival benefits of prophylactic lymph node dissection in patients without palpable inguinal lymph node have been reported. However, ILND indicated in case of palpable inguinal lymph nodes to achieve complete cure [3,5,7].
The role of radiotherapy as primary treatment has been described in few case reports but this is not a standard treatment at present. A recent trend is towards the use of multimodal therapy especially in late stage tumours (T3/T4 and high risk T2) to improve survival [5]. Our patient had locally advanced well-differentiated adenocarcinoma of the bulbomembranous urethra extended to prostatic urethra, invaded the corpus spongiosa with left inguinal lymphadenopathy and without distant metastasis (T2N1M0). So, multimodal therapy with surgery plus adjuvant chemotherapy was given. Chemotherapy for urethral adenocarcinoma is not so well defined. This patient was treated with chemotherapy regimen (5-FU+cisplatin) as for adenocarcinoma of other organs.
Recent survival analysis suggests that squamous cell cancer has more cancer specific survival than urothelial or adenocarcinoma (hazard ratio of 2.03 and 1.90, respectively) [5]. Overall survival for the bulbar urethral cancer is only 26% as compared to 69% in anterior UC [8]. Factors for the worse prognosis are advanced age, African-Americans, non-squamous histology, advanced stage of the tumour and presence of lymph nodes at the time of presentation [3].
Conclusion
Primary urethral adenocarcinoma in a male although very uncommon, but It should be considered as one of the differential diagnosis for any urethral mass, obstructive voiding and bleeding per urethra. Multimodal therapy should be given in advanced cases.
[1]. Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, Rare cancers are not so rare: The rare cancer burden in EuropeEur J Cancer 2011 47:2493-511. [Google Scholar]
[2]. Swartz MA, Porter MP, Lin DW, Weiss NS, Incidence of primary urethral carcinoma in the United StatesUrology 2006 68:1164-68. [Google Scholar]
[3]. Gakis G, Witjes JA, Compérat E, Cowan NC, De Santis M, Lebret T, Guidelines on Primary Urethral CarcinomaEuropean Association of Urology 2015 [Google Scholar]
[4]. Rabbani F, Prognostic factors in male urethral cancerCancer 2011 117:2426-34. [Google Scholar]
[5]. Sharp DS, Angermeier KW, Tumours of the urethra. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, editorsCampbell-Walsh Urology 2016 11th editionPhiladelphia, Pa, USASaunders:879-83. [Google Scholar]
[6]. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP, Management of primary urethral cancerUrology 1998 52(3):487-93. [Google Scholar]
[7]. Karnes RJ, Breau RH, Lightner DJ, Surgery for urethral cancerUrol Clin North Am 2010 37:445-57. [Google Scholar]
[8]. Dalbagni G, Zhang ZF, Lacombe L, Herr HW, Male urethral carcinoma: analysis of treatment outcomeUrology 1999 53:1126-32. [Google Scholar]