Introduction
Acne vulgaris is a common dermatosis of pilosebaceous units. It is characterized by seborrhea, comedones, papules, pustules, nodules and, sometimes, scarring [1].
It is a multifactorial disorder. Androgens, abnormal keratinocyte cornification, Propionibacterium acnes (P. acnes) colonization and proinflammatory mediators and cytokines all play roles in disease development [2].
LXR-α is a ligand activated transcription factor. It contains a central DNA-binding domain and a large ligand-binding domain with a lipophilic core that binds specific small-lipid molecules. After ligand binding, receptor undergoes a conformational change that promotes interaction with co-activator proteins that facilitate transcription of target genes [3].
LXR-α is recognized as sterol-responsive with the ability to bind to several oxysterolmetabolites. Many LXR-α target genes have been identified that implicate this receptor in a variety of physiological and pathological processes including cholesterol transport and metabolism, glucose metabolism and inflammation [4].
LXR-α is highly expressed in liver and is also found in adipose tissue, intestine, kidney and macrophages. It is also expressed in sebaceous glands, sweat glands and hair follicles [5].
Cyclo-oxygenase (COX) is a rate limiting key enzyme in the synthesis of Prostaglandins (PGs). It plays a role in certain biologic processes, including inflammation, angiogenesis, development and homeostasis [6].
There are two known forms of COX; COX1 which is present normally in all tissues and COX2 which is released by stimulatory cytokines during inflammation or infection [7].
COX2 is one of the downstream targets of NF-κB. It is induced in numerous processes such as cellular growth, differentiation, inflammation and tumourigenesis [8]. COX2 gene promoter contains several enhancer sequences and its expression can be induced through multiple signaling pathways [9]. The aim of this work was to evaluate the immunohistochemical expression of LXR-α and COX2 in acne vulgaris skin biopsies to explore their possible pathogenic role in this disease.
Materials and Methods
This case-control study was carried out on 65 subjects. These included 45 cases with AV that were selected from the Dermatology outpatient clinic, Faculty of Medicine, Menoufiya University Hospital, Egypt, during the period between March 2014 to April 2015 and 20 normal skin specimens from non acneic, age- and gender-matched subjects from the Plastic Surgery Department as a control group. The current work protocol was approved by The Ethical Committee of Menoufiya Faculty of Medicine.
A written consent form was obtained from every case and control subject before the study initiation. This was in accordance with the Helsinki Declaration of 1975 (revised in 2000).
All studied patients were subjected to complete history taking, general and dermatological examination. Clinical data describing patients’ demographics (age and gender) and disease duration were all documented. Disease duration was calculated from lesion onset to time of the first visit. Selected cases were newly diagnosed with no history of acne treatment. Disease aggravation by food intake, menstrual cycle or sun exposure was assessed for every case. Aggravation was defined as an increase of more than 25% in acne lesions compared to after exposure to specific factor [10].
Biopsied acne lesions were categorized into comedonal, papulopustular and nodular.
Clinical assessment of disease severity was done according to Tan JKL into [11]:
Mild acne: Fewer than 20 comedones or fewer than 15 inflammatory lesions or a total lesion count lower than 30.
Moderate acne: 20-100 comedones or 15-50 inflammatory lesions or a total lesion count of 30-125.
Severe acne: More than five nodules or comedones count greater than 100 or a total inflammatory count greater than 50 or a total lesion count greater than 125.
Scoring of AV lesions [
12]
Grade 1: Comedones and few papules;
Grade 2: Comedones, papules and a few pustules;
Grade 3: Larger inflammatory papules, pustules and a few cysts; a more severe form involving the face, neck and upper portions of the trunk;
Grade 4: More severe, with cysts becoming confluent.
Exclusion Criteria
Any case or control subject with one or more of the following was excluded:
Dermatological disease other than AV.
Systemic autoimmune or inflammatory diseases.
Local or systemic infections.
Biopsies
Skin biopsy samples were taken under 2% lignocaine local anesthesia from every case (from acne lesions and from perilesional skin) and control subject. Biopsies from cases and controls were site-matched. All specimens were fixed in 10% neutral-buffered formalin and subjected to routine tissue processing that ended with paraffin-embedded blocks ready for sectioning at the Pathology Department, Faculty of Medicine, Menoufiya University, Egypt.
Immunohistochemical (IHC) Staining for COX2 and LXR-α
5-μm-thick sections were cut from the paraffin-embedded blocks with subsequent steps of deparaffinization and dehydration in xylene and graded series of alcohol, respectively. Antigen retrieval was performed by boiling in 10 ml citrate buffer (pH 6.0) for 20 minutes, followed by cooling at room temperature. The slides were incubated overnight at room temperature with:
– Anti LXR-α concentrated Rabbit polyclonal antibody (ab106464) raised against LXR-α antigen, (Abcam Inc., Cambridge, USA).
– Anti COX2 concentrated Rabbit polyclonal antibody (RB-9072-PO) raised against COX2 antigen, (Thermo Fisher Scientific, USA).
Diaminobenzidine (DAB) was used as a chromogen. Slides were then counter-stained with Mayer’s hematoxylin.
Interpretation of LXR-α and COX2 immunohistochemical Results
A brown nucleo-cytoplasmic and cytoplasmic stain was considered positive for LXR-α [5] and COX2 [13] respectively in lesional, perilesional and control specimens.
Evaluation Included
Epidermis and pilosebaceous units were assessed for the following:
Expression either: Positive or negative
Percent of positive cells: The percentage of the positive cell was assessed at 200X magnification field [14].
Histo-score (H- score): H score was calculated to all positive specimens according to the following equation:
H score = +1x% of mildly stained cells + 2x% of moderately stained cells + 3x% of strongly stained cells [15].
Distribution was categorized as either:
– Patchy: irregular or not uniform distribution;
– Diffuse: uniform distribution.
Thickness pattern either:
– Partial thickness;
– Whole thickness.
Dermis was assessed for:
Inflammatory cell Expression: positive or negative.
Statistical Analysis
Data were collected, tabulated and statistically analysed using a personal computer with “(SPSS) version 11” program. Chi square test was used to study the association between qualitative variables. Mann–Whitney test was used for comparison between quantitative variables. Student’s t-test was used for comparison between two groups having quantitative variables. Spearman’s coefficient was used to study the correlation between two different variables. Differences were considered statistically significant with p<0.05.
Results
Clinical data of selected cases are summarized in [Table/Fig-1]. Immunohistochemical expression of COX2 in studied groups:
Clinical data of studied cases.
| Variables | Cases No= 45 |
|---|
| Age (years)Mean ± SDRange Median | 20.91±3.45616-3020 |
| Duration of disease (months)Mean ± SDRange Median | 3.8±2.51-105.5 |
| Variables | % | No (n) |
| Sex:MaleFemale | 40.060.0 | 1827 |
| Site of Acne:FaceBack Both | 40.015.644.4 | 18720 |
| Biopsied lesionComedonalPapulpustularNodular | 17.866.615.6 | 8307 |
| Exacerbating Factors:NoFood (chocolate, dairy products)MenstruationSun exposure | 57.824.611.16.5 | 261153 |
| Severity:Mild ModerateSevere | 42.251.16.7 | 19233 |
| Acne scoring system:1234 | 17.824.451.16.7 | 811233 |
| Family History:NegativePositive | 22.277.8 | 1035 |
SD: Standard deviation
COX2 Expression in Lesional Skin
All cases showed positive COX2 expression with variable distribution in epidermis and pilosebaceous units. Dermis showed positive expression in inflammatory cells in all cases [Table/Fig-2,3].
COX2 expression in studied groups.
| Variables | LesionalNo= 45 | PerilesionalNo= 45 | ControlNos=20 | p-value |
|---|
| COX2 expression in epidermis |
| COX2 %Mean ± SDMedian Range | 90.22 ± 10.9790.070-100 | 84.00±13.5580.050-100 | 70.50 ± 11.45970.050-90 | p1=0.001*p2=0.001*p3=0.029* |
| COX2 H-ScoreMean ± SDMedian Range | 145.33 ± 76.325100.070-300 | 108.89±45.2390.050-200 | 70.50 ± 11.45970.050-90 | p1=0.001*p2=0.001*p3=0.021* |
| No (%) | No (%) | No (%) | |
| COX2 DistributionDiffusePatchy | 39 (86.7)6 (13.3) | 39 (86.7)6 (13.3) | 9 (45.0)11 (55.0) | p1=0.001*p2=0.001*p3=1.00 |
| COX2 DistributionPartial thicknessWhole thickness | 18 (40.0)27 (60.0) | 29 (64.4)16 (35.6) | 15 (75.0)5 (25.0) | p1=0.001*p2=0.04**p3=0.02* |
| COX2 expression in pilosebaceous unit |
| COX2 %Mean± SDMedian Range | 86.89 ± 11.64390.070-100 | 77.33±17.10970.040-100 | 66.00 ± 11.42565.040-90 | p1=0.001*p2=0.009*p3=0.004* |
| COX2 H-scoreMean± SDMedian Range | 139.78 ± 70.630100.070-300 | 100.67±44.6980.040-200 | 66.00 ± 11.42565.040-90 | p1=0.001*p2=0.001*p3=0.005* |
| No (%) | No (%) | No (%) | |
| COX2 DistributionDiffusePatchy | 37 (82.2)8 (17.8) | 39 (86.7)6 (13.3) | 9 (45.0)11 (55.0) | p1=0.002*p2=0.001*p3=0.561 |
| COX2 DistributionPartial thicknessWhole thickness | 22 (48.9)23 (51.1) | 30 (66.7)15 (33.3) | 15 (75)5 (20) | p1=0.091p2=0.502p3=0.088 |
| COX2 expression in dermis |
| Inflammatory cellsNegativePositive | 045 (100.0) | 1 (2.2)44 (97.8) | 20 (100.0)0 | p1=0.001*p2=0.001*p3=1.000 |
SD: Standard deviation, p1: lesion vs control. p2: perilesion vs control. p3: lesion vs perilesion, *: Significant.
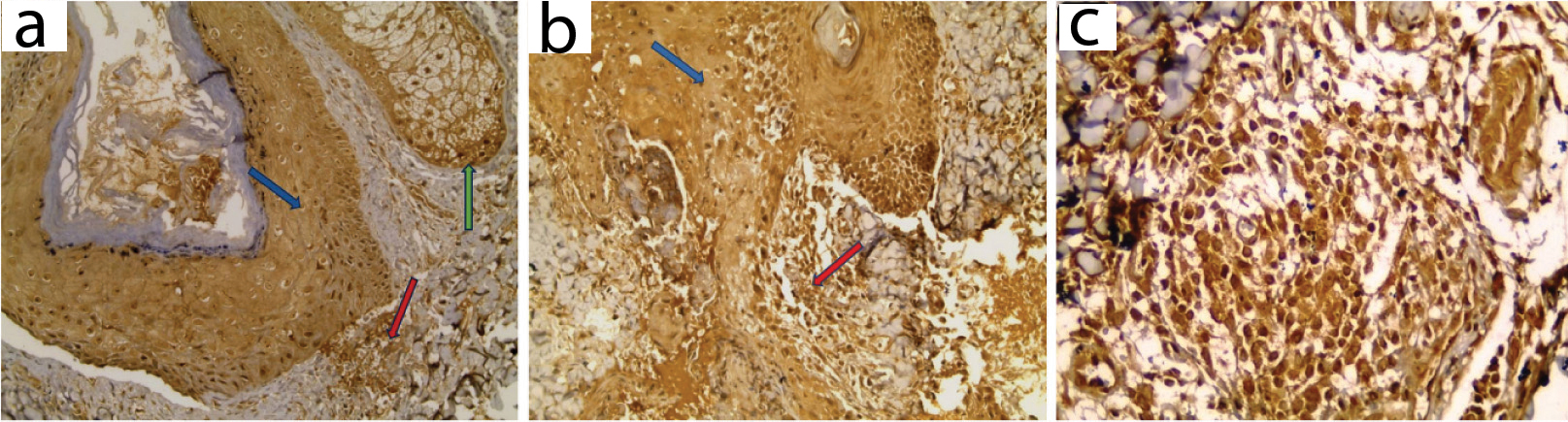
COX2 expression in lesional skin: a) Positive expression in hair follicle (blue arrow), sebaceous gland (green arrow) and dermal inflammatory cells (red arrow) in comedone; b) Positive expression in hair follicle and epidermal keratinocytes (blue arrow) and derma inflammatory cells (red arrow) in papule; c) Positive expression in dermal inflammatory cells in pustule (IHC X200 for a, b and X 400 for c).
COX2 Expression in Perilesional Skin
All cases showed positive COX2 immunostaining with variable distribution both in epidermis and pilosebaceous units. Dermis showed positive expression in inflammatory cells in 97.8% of cases [Table/Fig-2,4].
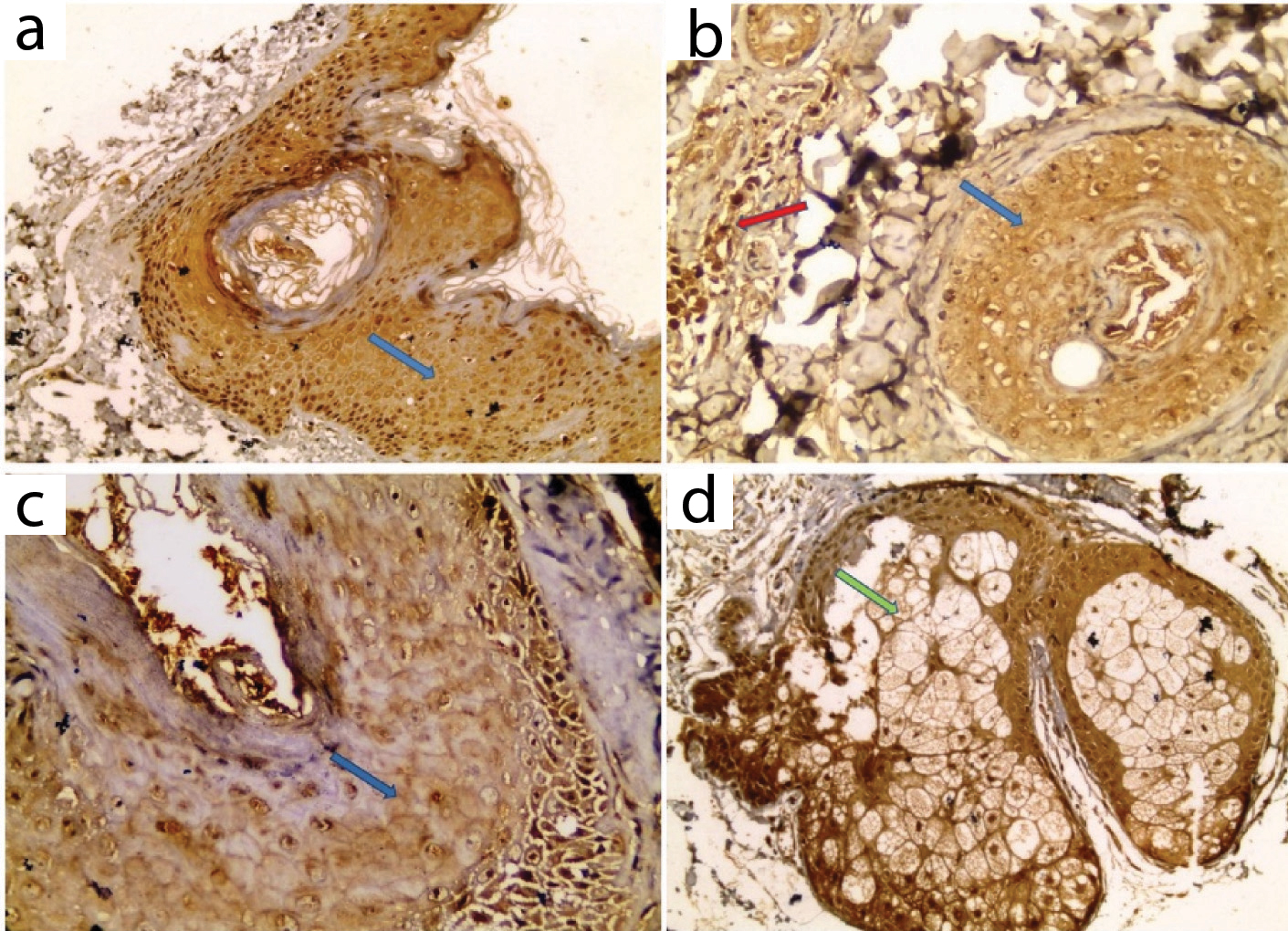
COX2 expression in perilesional skin: a) Positive expression in epidermis (green arrow) and inflammatory cells (blue arrows); b,c) Positive expression in hair follicle (green arrows); d) Positive expression in hair follicle (green arrow) and inflammatory cells (blue arrow) (IHC X200 for a, X400 for b, c, d).
COX2 Expression in Control Skin
All examined sections showed positive expression with variable distribution in epidermis and pilosebaceous units. Dermal expression was negative in all examined control samples [Table/Fig-2,5].
COX2 expression in control skin: a) Positive epidermal expression; b) Positive expression in sebaceous gland (IHC X200 for a, X400 for b).
Comparison between COX2 Expression in Studied Groups
Higher COX2 %, higher H score and diffuse distribution were significantly associated with lesional skin compared with control in epidermis and pilosebaceous units (p<0.001 for all). Whole thickness staining (p<0.001) in epidermis was significantly higher in lesional skin compared with control skin [Table/Fig-2].
Positive inflammatory cell expression (p<0.001) was significantly higher in lesional skin compared with control in the dermis [Table/Fig-2].
Higher COX2 % and higher H-score, were significantly associated with lesional skin compared with perilesional skin in epidermis (p=0.02 for both) and pilosebaceous units (p=0.004, p=0.005 respectively). Whole thickness staining (p=0.02) in epidermis was significantly higher in lesional skin compared with perilesional skin [Table/Fig-2].
Higher COX2 %, higher H-score and diffuse distribution, were significantly associated with perilesional skin compared with control in epidermis and pilosebaceous units (p<0.001 for all). Positive dermal inflammatory cell expression (p<0.001) was significantly higher in perilesional skin compared with control [Table/Fig-2].
Relationship between COX2% in Lesional Skin and Clinical Data of selected cases
Higher epidermal COX2 % was significantly associated with papulopustular acne (p=0.009) and higher acne score (p =0.018). Higher pilosebaceous units COX2 % was significantly associated with papulopustular acne (p=0.04) [Table/Fig-6].
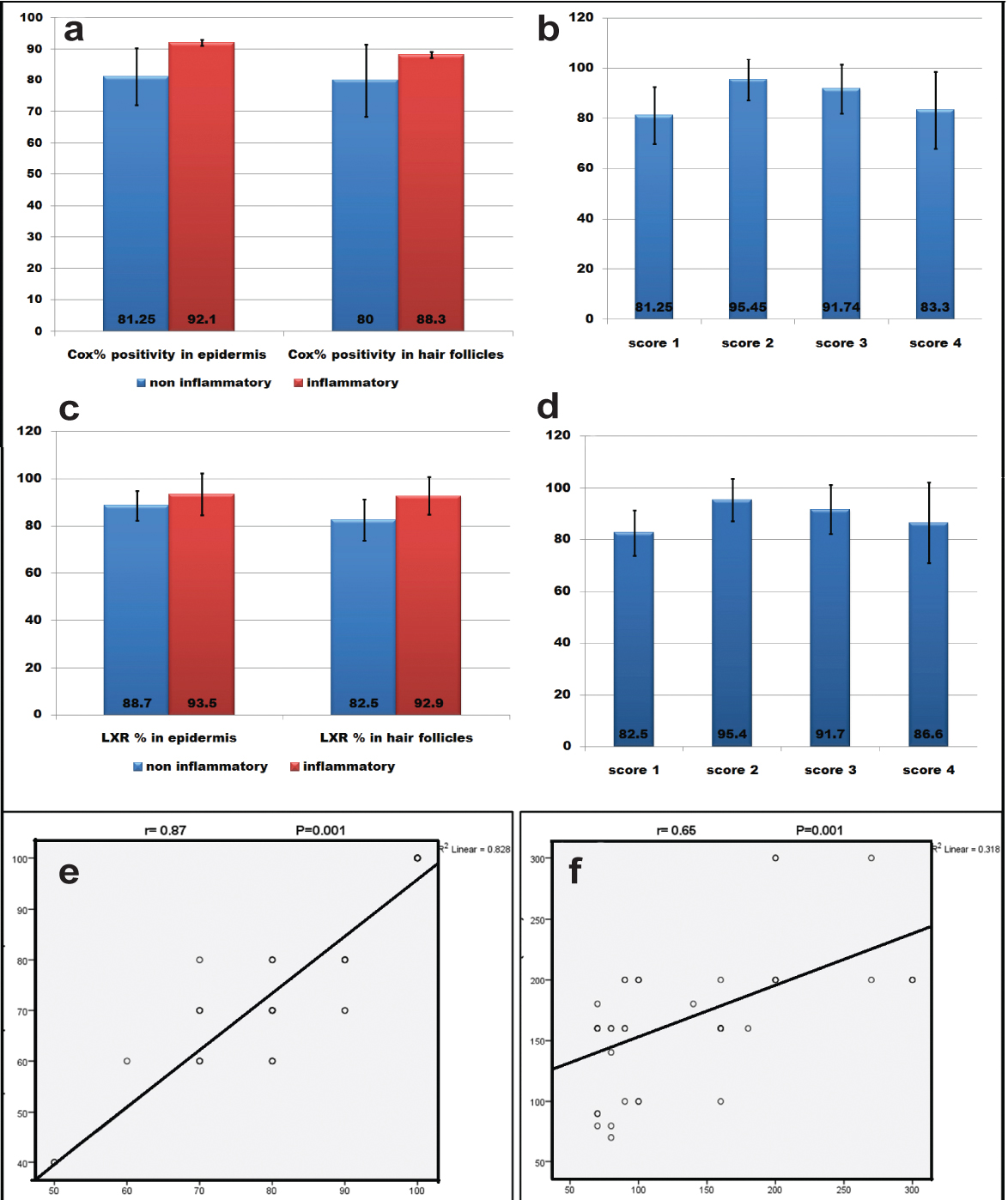
a) association between COX2% and AV lesions; b) association between COX2% and acne score; c) association between LXR-α % and AV lesions; d) association between LXR-α % and acne score; e) correlation between COX2 and LXR-% in epidermis; f) correlation between COX2 and LXR- % in pilosebaceous units.
Immunohistochemical Expression of LXR-α in Studied Groups
LXR-α expression in lesional skin: All cases showed positive LXR-α expression with variable distribution in epidermis and pilosebaceous units. Dermis showed positive expression in inflammatory cells in all cases [Table/Fig-7,8].
LXR-α expression in studied groups.
| Variable | LesionalNo= 45 | PerilesionalNo= 45 | ControlNo=20 | p-value |
|---|
| LXR-α expression in epidermis |
| LXR α %Mean± SDMedianRange | 92.67±8.634100.070-100 | 77.78±16.77270.040-100 | 70.50 ± 9.9870.050-90 | p1=0.001*p2=0.033p3=0.001* |
| LXR α H-ScoreMean± SDMedianRange | 190.89±60.784180.080-300 | 98.44±41.28480.040-200 | 70.50 ± 9.9870.050-90 | p1=0.001*p2=0.002*p3=0.001* |
| No (%) | No (%) | No (%) | |
| LXR α distributionDiffusePatchy | 39 (86.7)6 (13.3) | 39 (86.7)6 (13.3) | 10 (50)10 (50.0) | p1=0.004*p2=0.004*p3=1.00 |
| LXR α distributionPartialWhole thickness | 18 (40.0)27 (60.0) | 30 (66.7)15 (33.3) | 18 (90.0)2 (10.0) | p1=0.011*p2=0.048*p3=0.011* |
| LXR-α expression in pilosebaceous unit |
| LXR α %Mean± SDMedianRange | 90.67±10.313100.070-100 | 86.44±89.70070.030-660 | 64.00±10.4670.040-80 | p1=0.001*p2=0.099p3=0.001* |
| LXR α H-scoreMean± SDMedianRange | 170.00 ± 53.55160.070-300 | 89.78±37.74980.030-200 | 64.00±10.4670.040-80 | P1=0.001*P2=0.002*P3=0.001* |
| No (%) | No (%) | No (%) | |
| LXR α distributionDiffusePatchy | 39 (86.7)6 (13.3) | 39 (86.7)6 (13.3) | 10 (50)10 (50.0) | p1=1.00p2=0.004*p3=1.00 |
| LXR α distributionPartialWhole thickness | 18 (38.6)27 (61.4) | 30 (66.7)15 (33.3) | 18 (90)2 (10.0) | p1=0.008*p2=0.048*p3=0.008* |
| LXR-α expression in dermis |
| inflammatory cellsNegativePositive | 8 (7.8)37 (82.2) | 11 (24.4)34 (75.6) | 20 (100. 0)0 | p1=0.001*p2=0.001*p3=0.438 |
SD: Standard deviation, p1: lesion vs control. p2: perilesion vs control. p3: lesion vsperilesion, *: Significant.
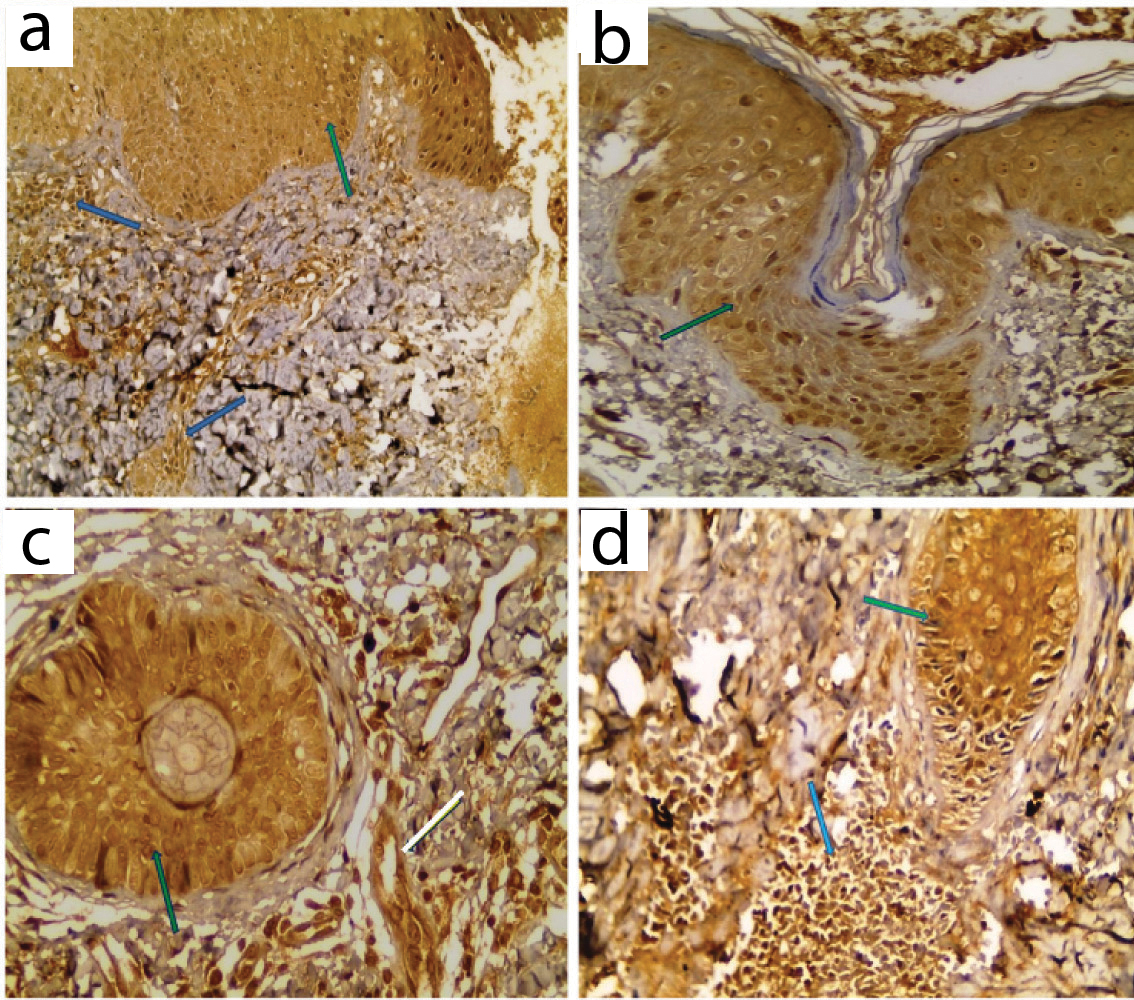
LXR-α expression in lesional skin: a) Positive patchy whole thickness expression in hair follicle (blue arrow) in comedone; b) Positive diffuse whole thickness expression in hair follicle (blue arrow) and inflammatory cells (red arrow) in papule; c) Positive patchy expression in hair follicle in comedone; d) Positive expression in hyperplastic sebaceous gland (IHC X 100 for a, X 200 for b, X400 for c,d).

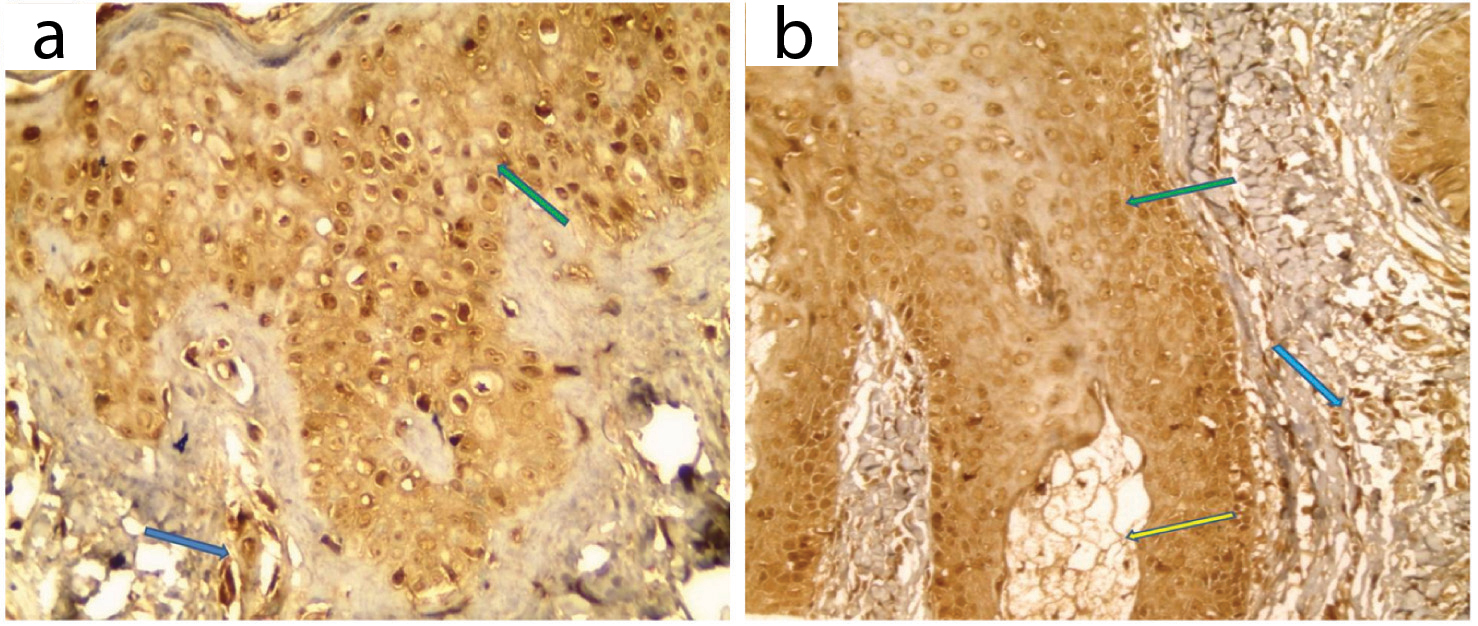
LXR-α expression in perilesional skin: All cases showed positive LXR-α immunostaining with variable distribution both in epidermis and pilosebaceous units. Dermis showed positive expression in inflammatory cells in all cases [Table/Fig-7,9].
LXR-α expression in perilesional skin: a) Positive patchy expression in epidermis (green arrow) and inflammatory cells (blue arrow); b) Positive patchy expression in hair follicle (green arrow), sebaceous gland (yellow arrow) and inflammatory cells (blue arrow) (IHC X400 for a,b).
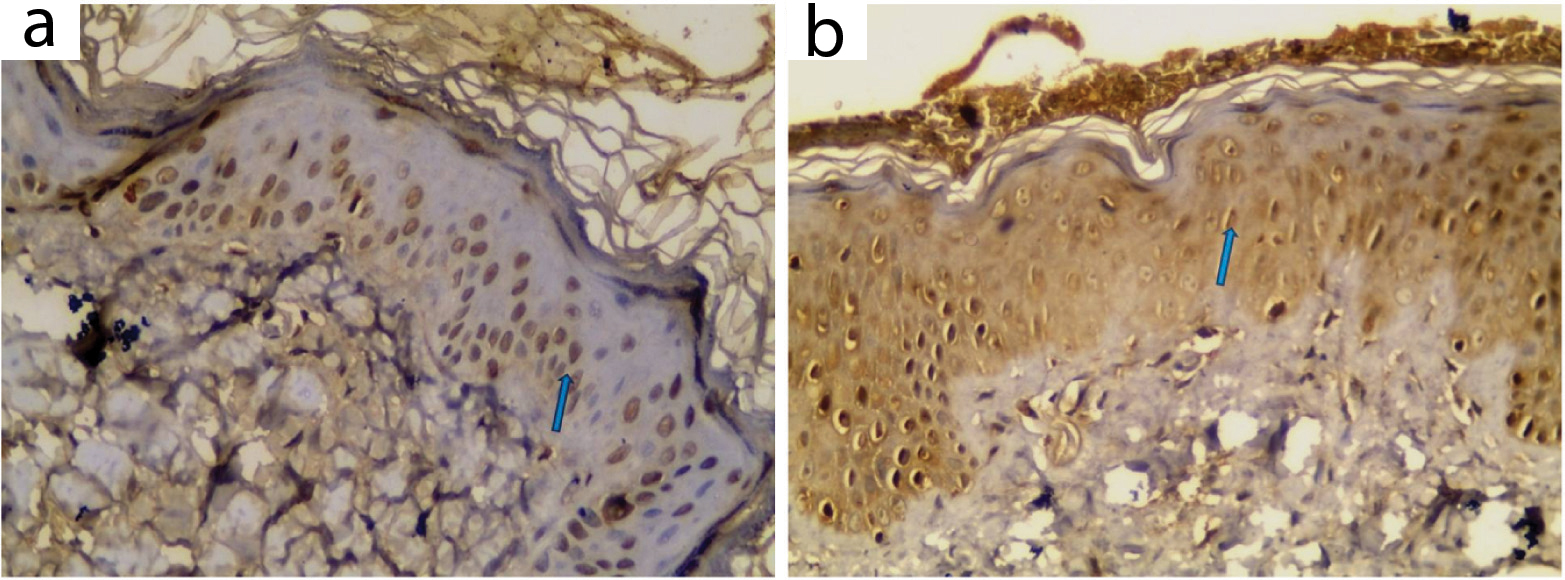
LXR-α expression in control skin: All examined sections showed positive expression with variable distribution in epidermis and pilosebaceous units. Dermal expression was negative in all examined control samples [Table/Fig-7,10].
LXR-α expression in control skin: a,b) Positive patchy epidermal expression (IHC X400 for a,b).
Comparison between LXR-α Expression in Studied Groups
Higher epidermal and pilosebaceous units% (p<0.001 for both), higher H-score (p<0.001 for both), diffuse distribution (p=0.004 for both) and whole thickness distribution (p<0.001 for both) were significantly associated with lesional compared with control skin. Positive expression in dermal inflammatory cells (p<0.001) was significantly higher in lesional compared with control skin [Table/Fig-7].
Higher epidermal and pilosebaceous units% (p<0.001 for both), higher H-score (p<0.001 for both) and whole thickness distribution (p=0.011) in epidermis were significantly associated with lesional skin compared with perilesional skin [Table/Fig-7].
Higher epidermal and pilosebaceous units H score (p=0.002 for both), diffuse distribution (p=0.004 for both) and whole thickness staining (p=0.04 for both) were significantly associated with perilesional skin compared with control. Positive expression in dermal inflammatory cells (p<0.001) was significantly higher in perilesional compared with control skin [Table/Fig-7].
Relationship between LXR-α % and Clinical Data of Studied Cases
Higher LXR-α% in epidermis and pilosebaceous units was significantly associated with papulopustular acne (p=0.01) and higher acne score (p=0.03) [Table/Fig-6].
Significant positive correlation was detected between COX2% and LXR-α% in epidermis (p=0.001, r=0.87) and pilosebaceous units (p=0.001, r=0.65) [Table/Fig-6].
Discussion
In the present work, LXR-α expression was positive in epidermis of all control group with whole thickness staining in 50% of sections. This was in agreement with Hanley K et al., who reported that LXR-α is expressed in cultured human keratinocytes throughout all layers of the human epidermis [16].
LXRs may play an important role within the skin. In vivo and in vitro studies showed that LXR ligands share in stratum corneum formation and induce down-regulation of cell proliferation. These ligands stimulate cornified envelope formation through increased transcription of transglutaminase 1, involucrin, loricrin and filaggrin [17].
In the present work, LXR-α expression was positive in hair follicles and sebaceous glands of all control group. This was in agreement with Komuves LG et al., and Hong I et al., [17,18].
It was found that activation of LXR-α induces lipid synthesis in sebocytes. Additionally, it induces Sterol Regulatory Element-Binding Protein 1 (SREBP-1) that regulates genes required for fatty acid and lipid metabolism and production [18].
In hair follicles, LXR-α has been shown to be expressed not only in the outer root sheath but also in the dermal papilla, connective tissue sheath, and hair bulb. This pattern of expression suggests an important role in mediating hair follicle differentiation programs [19].
The current study showed that LXR-α was upregulated in lesional AV skin compared with perilesional skin and control group providing evidence of its role in disease pathogenesis.
As mentioned earlier, LXR-α plays vital roles in lipid metabolism and cholesterol homeostasis. In the human epidermis, cholesterol contributes in the formation of the permeability barrier via lamellar body formation [20].
It has been reported that male and female acne patients have significantly elevated total cholesterol and low-density lipoprotein cholesterol [21,22].
LXR-α acts as cholesterol sensor; when cellular oxysterol accumulates as a result of increasing concentrations of cholesterol, LXR-α induces the transcription of genes such as the ATP-Binding Cassette (ABCA1), leading to cholesterol efflux thus lowering intracellular cholesterol [23] protecting cells from cholesterol overload [24,25].
It has been reported that lipogenic enzymes such as fatty acid synthase are regulated by the sterol regulatory binding protein-1 which is regulated by liver LXR-α [26]. LXR-α agonists were shown to increase triglyceride accumulation and to promote lipogenesis [27].
Acne patients have increased sebum production and there is positive correlation between acne severity and rate of sebum production [28]. Lipoperoxides and monounsaturated fatty acids affect keratinocyte proliferation and keratinization. Peroxides are proinflammatory and stimulate the release of inflammatory cytokines [29].
Therefore, it is assumed that the effects of LXR-α on the induction of acne are related to control of cellular cholesterol metabolism inducing excess lipogenesis, which promotes the progress of the disease [30].
The current work showed that, LXR-α was upregulated in perilesional skin compared with control skin. It was reported that inflammatory events begin in perilesional skin in the early stage of the disease even before hyperproliferation or abnormal cornification of follicular keratinocytes. Inflammatory micro-environment is present in perilesional area before evident clinical lesions appear [31].
LXR-α is an important regulator of inflammatory gene expression and innate immunity [32]. Topical treatment with LXR-α endogenous and synthetic agonists have potent anti-inflammatory activity in cutaneous inflammation by inhibition of cytokine production [33]. In addition, LXR-α inhibits the expression of macrophage inflammatory genes, including inducible Nitric Oxide Synthase (iNOS), COX-2, interleukin (IL-6), IL-1β, Monocyte Chemo-attractant Protein-1 (MCP-1) and MCP-3, in response to bacterial, Tumour Necrosis Factor-α (TNF-α) or lipopolysaccharide stimulation [34,35]. This role may explain the positive LXR-α expression by inflammatory cells in lesional and perilesional skin, noted in the present study.
The current study demonstrated significantly higher LXR-α% in papulopustular AV than comedonal lesions.
This was in agreement with Bosseila M et al., and may indicate that LXR-α may play a role in the progression of disease from comedonal to papulopustular lesions [36,37] through its role in cholesterol metabolism and lipogenesis, as mentioned earlier [30].
In the present work, positive diffuse COX2 expression was present in epidermis of all control specimens. This was in agreement with Ikai K [38].
Others detected restricted COX2 expression to keratinocytes of the granular and spinous layers [39,40]. However, An KP et al., reported complete absence of COX2 expression in normal skin [41].
COX2 expression and its role in normal epidermis are controversial issues. Scholz K et al., postulated that COX2 expression is part of normal human keratinocyte differentiation [42]. An increasing intensity of expression is observed as one moves from the suprabasilar stratum spinosum of the epidermis to the stratum granulosum where the signal is most intense [43]. However, Ikai K concluded that, the positive expression in basal and suprabasal cell layers may provide evidence about its role in normal keratinocyte proliferation as well as differentiation [38]. Findings of the present work underscore the possible role of COX2 in cell proliferation and differentiation.
This role can be explained through the effects of mediators produced by COX2 activation. Cutaneous Prostaglandin E2 (PGE2) is produced by keratinocytes and fibroblasts. It has proinflammatory, proproliferative and immunomodulatory effects [44].
PGE2 trigger the generation of inflammatory mediators, and the latter enhance the local leukotriene and PG production. Even if PGE2 would not only be mainly formed by sebocytes in acne, but also from the participating inflammatory cells, the final result would be identical [45].
In addition, in the mouse, transgenic overexpression of COX2 in the basal compartment of the epidermis correlating with increased PGE2 levels caused sebaceous gland hyperplasia and overshooting sebum production, pointing to a possible role of COX2-mediated PGE2 synthesis in sebum production [46]. However, the exact sequence of events regarding the interaction of human sebaceous gland cells with inflammatory cells or other agents leading to the induction of PG pathways in acne-involved sebaceous glands is not known [45].
The current study showed positive COX2 expression in sebaceous glands in control skin. This was in agreement with Alestas T et al., and Zhang Q et al., [45,47].
The positive hair follicle expression of COX2, demonstrated in the current work, was previously reported by Müller-Decker K et al., who stated that COX2 expression is hair cycling-dependent, becoming apparent in elongated hair germs, later on in the Outer Root Sheath (ORS) of the distal and proximal hair follicles, declining in catagen and telogen, and then being re-induced in the ORS of anagen hair follicles [48].
The current study showed positive COX2 expression in epidermis and pilosebaceous follicles in all cases of acne vulgaris. Furthermore, COX2 was up regulated in acne lesions compared with perilesional and normal skin. This supports the fact that increased COX2 expression may have a role in acne pathogenesis. This was in agreement of Aletas T et al., and Neufang G et al., [45,46].
Ottaviani M et al., reported increased expression of COX2 and PGE2 in acne involved skin associated with enhanced release of pro-inflammatory cytokines and a higher degree of lipoperoxidation. Authors stated that their findings support the interplay between lipoinflammation and lipid signaling in AV development [28].
All cases of acne vulgaris examined in the current study showed positive COX2 expression by dermal inflammatory cells. There was significant association between COX2 % in (lesional epidermis and pilosebaceous units) and papulopustular acne.
This role can be explained through the effects of mediators produced by COX2 activation. In response to an inflammatory insult, the release of PGs, and more importantly PGE2, constitutes a key event in the development of inflammation [49].
The presence of inflammation is the critical link between acne and eicosanoids. Sebaceous gland seems to be the key tissue in this relationship [50,51].
The current study showed that there was higher COX2% in epidermis pilosebaceous units in perilesional skin more than control. There was also higher COX2 positivity in inflammatory cells in perilesional skin more than control. This could be explained by the presence of inflammatory microenvironment in perilesional skin even before the apparent clinical disease [31].
In the current study, there was significant positive correlation between COX2% and LXR-α % and between COX2 H-score and LXR-α H-score in lesional epidermis and pilosebaceous units.
Contrary to our results, Fowler AJ et al., stated that activation of LXR-α inhibited the expression of COX2 in the SZ95 sebocytes [33]. It was reported that LXR-α pathway antagonizes inflammatory gene expression [52,53].
Now, a question arises; what is the therapeutic value of LXR-α and COX2 antagonists in AV treatment?
Hong I et al., documented that LXR-α antagonists could be clinically implicated for the treatment of seborrhea and acne [18]. Viennois E et al., stated that selective LXR-α agonists that are isoform and tissue-specific could provide localized and specific cutaneous effects that do not influence other LXR receptor pools [54]. Clinical trials using COX2 inhibitors in management of AV are needed.
Limitation
The study was carried out on a small number of cases who were of the same ethnic background. Future large-scaled research on population of different ethnicities including other AV variants and acneform eruptions is needed.
Conclusion
Both LXR-α and COX-2 play a role in the pathogenesis of acne vulgaris through their effects on cellular proliferation, inflammation and lipid synthesis. Research for new therapeutic modalities for acne vulgaris based on their inhibition is needed. More understanding of the interaction between LXR-α, COX2 and acne lesions may lead to effective therapies, possibly directed toward specific cell types or steps within the inflammatory pathways.
SD: Standard deviation
[1]. Adityan B, Kumari R, Thappa DM, Scoring systems in acne vulgarisIndian J Dermatol Venereol Leprol 2009 75:323-26. [Google Scholar]
[2]. Harper JC, Thiboutot DM, Pathogenesis of acneAdv Dermatol J 2003 19:1-10. [Google Scholar]
[3]. Edwards PA, Kennedy MA, Mak PA, LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasisVascul Pharmacol 2002 38:249-56. [Google Scholar]
[4]. Michael Laura F, Schkeryantz JM, Burris TP, The pharmacology of LXRMini Rev Med Chem 2005 5:729-40. [Google Scholar]
[5]. Russell LE, Harrison WJ, Bahta AW, Zouboulis CC, Burrin JM, Philpott MP, Characterization of Liver X receptor expression and function in human skin and the pilosebaceousExperim Dermatol J 2007 16:844-52. [Google Scholar]
[6]. Williams CS, Mann M, DuBois RN, The role of cyclooxygenases in inflammation, cancer, and developmentOncogene 1999 18:7908-16. [Google Scholar]
[7]. Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Interleukin-1 ß mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivityNature 2001 410:471-75. [Google Scholar]
[8]. Simon L, Role and regulation of cyclooxygenase-2 during inflammationAm J Med 1999 106:37-42. [Google Scholar]
[9]. Smith W, Garavito R, Dewitt D, Prostaglandin endoperoxide H synthase (cyclooxygenases-1 and-2)J Bio Chem 1996 271:33157-60. [Google Scholar]
[10]. Jung JY, Yoon MY, Min SU, Hong JS, Choi YS, Suh DH, The influence of dietary patterns on acne vulgaris in KoreansEur J Dermatol 2010 20:768-72. [Google Scholar]
[11]. Tan JKL, Current measures for the evaluation of acne severity: Methods of grading acne severityExpert Rev Dermatol 2008 3:595-603. [Google Scholar]
[12]. Witkowski JA, Parish LC, The assessment of acne: An evaluation of grading and lesion counting in the measurement of acneClin Dermatol 2004 22:394-97. [Google Scholar]
[13]. Bakry OA, Samaka RM, Shoeib MA, Abdel Aal SM, Nuclear factor kappa B and cyclo-oxygenase-2: two concordant players in psoriasis pathogenesisUltrastruct Pathol 2015 39:49-61. [Google Scholar]
[14]. Bahnassy AA, Zekri AR, El-Houssini S, El-Shehaby AM, Mahmoud MR, Abdallah S, Cyclin A and cyclin D1 as significant prognostic markers in colorectal cancer patientsBMC Gastroenterol 2004 4:22-23. [Google Scholar]
[15]. Bilalovic B, Sandstad R, Golouh H, El-Shehaby AM, Mahmoud MR, Abdallah S, CD10 protein expression in tumour and stromal cells of malignant melanoma is associated with tumour progressionMod Pathol 2004 17:1251-58. [Google Scholar]
[16]. Hanley K, Komuves LG, Bass NM, He SS, Jiang Y, Crumrine D, Fetal Epidermal differentiation and barrier development in vivo is accelerated by nuclear hormone receptor activatorsJ Invest Dermatol 1999 113:788-95. [Google Scholar]
[17]. Komuves LG, Schmuth M, Fowler AJ, Elias PM, Hanley K, Man MQ, Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermisJ Invest Dermatol 2002 118:25-34. [Google Scholar]
[18]. Hong I, Lee MH, Na TY, Zouboulis CC, Lee MO, LXR alpha enhances lipid synthesis in SZ95 sebocytesJ Invest Dermatol 2008 128:1266-72. [Google Scholar]
[19]. Nguyen H, Rendl M, Fuchs E, Cell Tcf3 governs stem cell features and represses cell fate determination in skinJ Immunol 2006 127:171-83. [Google Scholar]
[20]. Schurer NY, Elias PM, The biochemistry and function of stratum corneum lipidsAdv Lipid Res 1991 24:27-56. [Google Scholar]
[21]. El-Akawi Z, Abdel-Latif N, Abdul-Razzak K, Al Aboosid M, The relationship between blood lipids profile and acneJ Health Sci 2007 53:596-99. [Google Scholar]
[22]. Abulnaja KO, Changes in the hormone and lipid profile of obese adolescent Saudi females with acne vulgarisBraz J Med Biol Res 2009 42:501-05. [Google Scholar]
[23]. Jiang YJ, Lu B, Kim P, Elias PM, Feingold KR, Regulation of ABCA1 expression in human keratinocytes and murine epidermisJ Lipid Res 2006 47:2248-58. [Google Scholar]
[24]. Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Accumulation of dietary cholesterol caused by mutations in adjacent ABC transportersSci 2000 290:1771-75. [Google Scholar]
[25]. Repa JJ, Mangelsdorf DJ, The liver X receptor gene team: potential new players in atherosclerosisNat Med 2002 8:1243-48. [Google Scholar]
[26]. Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptorsJ Biol Chem 2002 277:11019-25. [Google Scholar]
[27]. Darimont C, Avanti O, Zbinden I, Leone-Vautravers P, Mansourian R, Giusti V, Liver X receptor preferentially activates de novo lipogenesis in human preadipocytesBiochem 2006 3(4):309-18. [Google Scholar]
[28]. Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M, Peroxidatedsqualene induces the production of inflammatory mediators in HaCaT keratinocytes: A possible role in acne vulgarisJ Invest Dermatol 2006 126:2430-37. [Google Scholar]
[29]. Smith RN, Braue A, Varigos GA, Mann NJ, The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglyceridesJ Dermatol Sci 2008 50:41-52. [Google Scholar]
[30]. Hong C, Tontonoz P, Coordination of inflammation and metabolism by PPAR and LXR nuclear receptorsCurr Opin Genet Dev 2008 18:461-67. [Google Scholar]
[31]. Jeremy AHT, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ, Inflammatory events are involved in acne lesion initiationJ Invest Dermatol 2003 121:20-27. [Google Scholar]
[32]. Zelcer N, Tontonoz P, Liver X receptors as integrators of metabolic and inflammatory signalingJ Clin Invest 2006 116:607-14. [Google Scholar]
[33]. Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine productionJ Invest Dermatol 2003 120:246-55. [Google Scholar]
[34]. Bruemmer D, Law RE, Liver x receptors: potential novel targets in cardiovascular diseasesCurr Drug Targets Cardiovasc Haematol Disord 2005 5:533-40. [Google Scholar]
[35]. Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P, Reciprocal regulation of inflammation and lipid metabolism by liver X receptorsNat Med 2003 9:213-19. [Google Scholar]
[36]. Bosseila M, Tawfic SO, Ezzat MA, Shaker OG, Tissue liver X-receptor-α (LXRα) level in acne vulgarisJ Egy Wom Dermatol Soc 2013 10:101-05. [Google Scholar]
[37]. Do TT, Zarkhin S, Orringer JS, Nemeth S, Hamilton T, Sachs D, Computer-assisted alignment and tracking of acne lesions indicate that most inflammatory lesions arise from comedones and de novoJ Am Acad Dermatol 2008 58:603-08. [Google Scholar]
[38]. Ikai K, Psoriasis and the arachidonic acid cascadeJ Dermatol Sci 1999 21:135-46. [Google Scholar]
[39]. Leong J, Hughes-Fulford M, Rakhlin N, Habib A, Maclouf J, Goldyne ME, Cyclooxygenases in human and mouse skin and cultured human keratinocytes: association of COX-2 expression with human keratinocyte differentiationExp Cell Res 1996 224:79-87. [Google Scholar]
[40]. Kagoura M, Toyoda M, Matsui C, Khodaeiani E, Alikhah H, Naghavi-behzad M, Immuno-histochemical expression of cyclooxygenase-2 in skin cancersJ Cutan Pathol 2001 28:298-302. [Google Scholar]
[41]. An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Cyclooxygenase-2 expression inmurine and human nonmelanoma skin cancers: implications for therapeutic approachesPhotochem Photobiol 2002 76:73-80. [Google Scholar]
[42]. Scholz K, Furstenberger G, Muller-Decker K, Marks F, Differential expression of prostaglandin-H synthase isoenzymes in normal and activated keratinocytes in vivo and in vitroBiochem J 1995 309:263-69. [Google Scholar]
[43]. Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinasesJ BiolChem 1999 274:29138-48. [Google Scholar]
[44]. Rhodes LE, Gledhill K, Masoodi M, Haylett AK, Brownrigg M, Thody AJ, The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phasesFASEB J 2009 23:3947-56. [Google Scholar]
[45]. Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC, Enzymes involved in the biosynthesis of leukotriene B(4) and prostaglandin E(2) are active in sebaceous glandsJ Mol Med 2006 84:75-87. [Google Scholar]
[46]. Neufang G, Furstenberger G, Heidt M, Marks F, Müller-Decker K, Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skinProc Natl Acad Sci U S A 2001 98:7629-34. [Google Scholar]
[47]. Zhang Q, Seltmann H, Zouboulis CC, Konger RL, Involvement of PPAR gamma in oxidative stress-mediated prostaglandin E(2) production in SZ95 human sebaceous gland cellsJ Invest Dermatol 2006 126:42-48. [Google Scholar]
[48]. Müller-Decker K, Leder C, Neumann M, Neufang G, Bayerl C, Schweizer J, Expression of cyclooxygenase isozymes during morphogenesis and cycling of pelage hair follicles in mouse skin: precocious onset of the first catagen phase and alopecia upon cyclooxygenase-2 overexpressionJ Invest Dermatol 2003 121:661-8. [Google Scholar]
[49]. Hecker M, Foegh ML, Ramwell PW, The eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes and related compoundsIn: Basic and Clinical Pharmacology; by, Katzung BG (Ed) 1995 Norwalk, CTAppleton & Lange:290-304. [Google Scholar]
[50]. Fitzgerald GA, Arachidonic acid is a bioactive moleculeJ Clin Invest 2001 107:1339-45. [Google Scholar]
[51]. Laneuville O, Breuer DK, Xu N, Huang ZH, Gage DA, Watson JT, Lagarde M, DeWitt DL, Smith WL, Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)- octadecatrienoic acids from alpha-linolenic acidJ Biol Chem 1995 18(270):19330-6. [Google Scholar]
[52]. Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P, Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophagesJ Biol Chem 2003 278:10443-49. [Google Scholar]
[53]. Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, LXR-dependent gene expression is important for macrophage survival and the innate immune responseCell 2004 119:299-309. [Google Scholar]
[54]. Viennois E, Mouzat K, Dufour J, Morel L, Lobaccaro JM, Baron S, Selective liver X receptor modulators (SLiMs): what use in human health?Mol Cell Endocrinol 2012 351:129-41. [Google Scholar]