Ameloblastoma is a locally invasive, slowly growing odontogenic neoplasm that has a high recurrence rate [1]. The invasion of adjacent healthy tissue by the neoplastic cells is an essential step in tumour advancement and this is supported in part by angiogenesis stimulated by stromal macrophages [2]. Neovascularization and MVD adjacent to ameloblastoma islands can be evaluated using the CD34, which is a sensitive marker of vascular endothelium [3]. CD34 staining is stronger and has a lower error rate when compared to other vascular markers [4].
The tumour microenvironment comprises numerous signaling molecules and pathways that influence the angiogenic response [5]. Angiogenesis can be stimulated by TAMs. TAMs are macrophages that have been modified in the milieu of the tumour microenvironment. These macrophages engage in complex interaction with stroma cells and thus modulate angiogenesis [6], tumour invasion and metastasis [7]. TAMs have lost host innate immune response ability and also have very weak or no ability to present antigens [8]. Thus, there is collaboration between the tumour and the tumour microenvironment to maintain tumour enlargement. TAMs exist in two phenotypically and functionally distinctive states: one is the classically activated (M1) state and the other is the otherwise activated (M2) state {these mirror the T helper (Th) 1 and 2 cells}. M1 macrophages possess antitumour activity, whereas M2 macrophages support tumour invasion and metastasis [9,10]. Anti-CCR7 antibody is a highly specific marker of M1 while CD206 is highly specific for M2 macrophages [11], and its increased expression was significantly associated with poor overall survival in various cancers [9,12].
We investigated the relative expression and topography of TAMs and CD34 in ameloblastoma to assess their affiliation and effect on tumour growth.
Materials and Methods
This was an in vitro study. Forty-six FFPE blocks of ameloblastoma cases from the Oral Pathology Department of the University College Hospital, University of Ibadan, Nigeria, were sectioned and stained with hematoxylin and eosin for re-evaluation and inclusion. At the Frankfurt Orofacial Regenerative Medicine (FORM) Lab, Department for Oral, Cranio-Maxillofacial and Facial Plastic Surgery, Medical Center of the Goethe University Frankfurt, Frankfurt am Main, Germany, all the FFPE blocks were each cut into three sections, de-paraffinized using xylene and hydrated with alcohol. The tissue were immersed in heat-induced epitope retrieval 10 mMol citrate buffer pH 6.0 (TA-250-PM1X), diluted 1:100 with distilled water and incubated at 95°C for 20 minutes. They were cooled in the buffer for 20 minutes and then rinsed in PBS for 5 minutes. Positive controls came with the kits and for negative controls we omitted the step of antibody application in the process. Thermo-Scientific peroxidase blocking reagent was added to each section for 15 minutes, and the sections were rinsed in 0.1% TBST for 5 minutes. The specimens were incubated for 60 minutes with the antibodies; Abcam Mouse monoclonal Anti-CCR7 antibody {Y59} (ab32527) {dilution 1:1000}, Abcam Rabbit polyclonal Anti-CD206 antibody {anti-mannose receptor antibody} (ab64693) {dilution 1:1000}, Dako Mouse monoclonal Anti-CD34 antibody QBEnd-10 (M7165) {dilution 1:25}. They were then rinsed with TBST, followed by incubation with pre-diluted (ready-to-use) UltraVision Quanto Detection System/Horse Radish Peroxidase for 15 minutes. An appropriate volume of 1 ml of diaminobenzidine substrate with one drop of diaminobenzidine chromogen was added to cover the slides, followed by incubation in a humidity chamber for 5 minutes. The sections were then immersed in aqueous Gill’s hematoxylin for 10 seconds and rinsed in distilled water for 5 minutes. The tissue was dehydrated and subsequently rinsed with xylene. DPX was applied, and a cover slip placed. Cytoplasmic/membrane brown staining was taken as positive for CCR7 and CD206 while cell membrane/cytoplasmic brown staining was considered as positive for CD34.
Quantification and scoring of CCR7 and CD206: Stained tissue sections were analysed using a semiquantitative method with a light microscope at X400 magnification. Three representative High Power Fields (hpf) showing maximum immunopositivity adjacent to tumour cells were selected for scoring by cell counting. The relative percentage of CCR7+ and CD206 + cells in relation to overall cellularity was calculated for each hpf, and the final score was taken as an average of the three hpf. Final scores for CCR7 and CD206 were classified as: <5%, 5-25%, 25-50% and >50% [13].
Quantification and scoring of CD34: TAMs related MVD was evaluated by choosing three fields, with the highest number of vessels at X10, adjacent to the TAMs to assess association of the vessels to the macrophages. Then, counting the CD34 positive vessels at X40 magnification, the MVD was calculated as the mean of the three-recorded values. Cases that had no CD34 + vessels adjacent to the TAMs region were regarded as having a MVD score of 0.
All slides were viewed with a Nikon ECLIPSE 80i microscope (Nikon, Tokyo, Japan) and microphotographs of the stained sections were recorded with a connected digital camera DS-Fi1 together with a Nikon digital sight control unit (Nikon, Tokyo, Japan).
This research investigation adhered strictly to the ethical standards of our Institutional Review Board and in accordance with the Helsinki’s declaration.
We determined to measure relative differential intratumoural and peritumoural topography. Hence, our results can be interpreted based on topography and some conclusions drawn. The sample size was determined by convenient sampling technique and since it is documented that benign clinical or histologic types of ameloblastomas do not have prognostic importance, we did not categorize the cases into types in this assessment.
Statistical Analysis
Simple descriptive statistics was applied.
Results
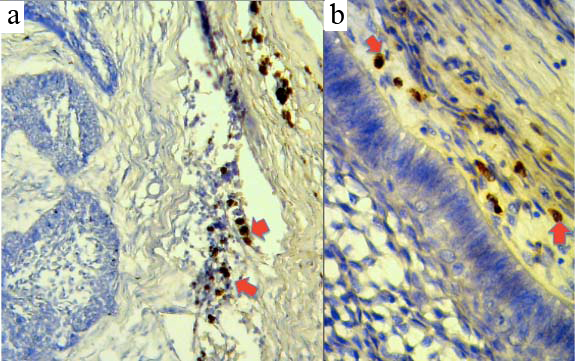
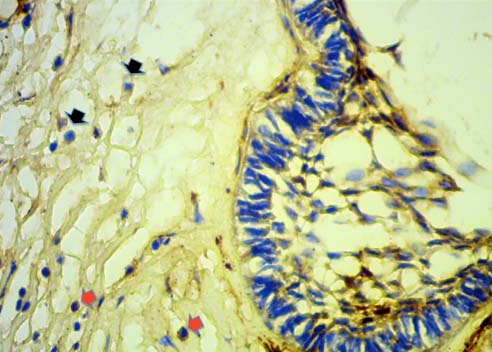
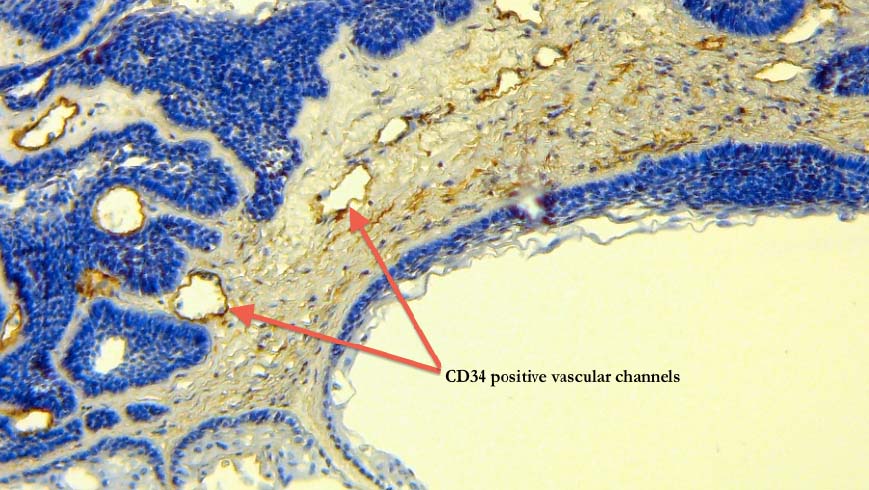
The macrophages adjacent to peritumour islands were marked by CD206 and CCR7, however CCR7 also slightly marked a region of the neoplastic cells [Table/Fig-1a,b, 2]. We noted only negligible intratumour presence of positive immune macrophages. The percentage of positive CCR7 immune cells was greater than that for CD206 in 38 (82.6%) cases, approximately equal to CD206 in 6 (13%) cases, and the CD206 expression was more than CCR7 in only 2 (4.3%) cases. Twenty-six (56.5%) cases of ameloblastoma had negative CD206 score among the population of immune cells, 16 (34.8%) cases expressed CD206 in <5% of immune cells present, while 4 (8.7%) cases expressed CD206 in 5-25% of immune cells. In 34 (73.9%) cases, the area of MVD did not overlap with the region of TAMs but in 4 (8.7%) cases (where MVD overlapped TAM1) the average MVD score was 20 for regions where TAMs positivity was >50% [Table/Fig-3,4].
CD206+ immune cells (marked by red arrows) adjacent to islands of odontogenic epithelium; in a) (X40) and b) (X400).
CCR7+ immune cells (marked by red arrows) and CCR7 negative immune cells (marked by black arrows) adjacent to islands of odontogenic epithelium X400.
CD34 positive vascular channels (marked by red arrows) adjacent to islands of odontogenic epithelium (X40).
Relative topographical expression of TAMs and MVD in ameloblastoma.
| TAMs + relative percentage score (%) | Cases with CCR7+ immune cells | Cases with CD206+ immune cells | Cases with MVD and TAMs overlapping | Average MVD score in areas related to TAMs |
|---|
| Nil | 1 (2.2%) | 26 (56.5%) | 34 (73.9%) | Nil |
| <5 | 12 (26.1%) | 16 (34.8%) | 1 (2.2%) | 12 |
| 5-25 | 10 (21.7%) | 4 (8.7%) | 3 (6.5%) | 10 |
| 25-50 | 9 (19.6%) | - | 4 (8.7%) | 13 |
| >50 | 14 (30.4%) | - | 4 (8.7%) | 20 |
Discussion
The relative expression of CCR7 far exceeds that of CD206 in comparable regions adjacent to islands of ameloblastoma in this study. This suggests a higher presence of TAM1 and hence greater anti-tumour activity, than TAM2 that has pro-tumour effects in the tumour. This may be explained by the fact that although locally aggressive, ameloblastoma is a benign tumour, and the surrounding microenvironment is able to recognize and mount substantial anti-tumour activity against it. On the other hand, malignant tumours typically have the TAM2 presence higher than TAM1, especially in poor prognostic cases [14].
We noted in this study that the TAMs were mostly seen at the peritumoural areas and only negligible intratumour presence of positive TAMs was seen. A study by Lin JY et al., described the density of peritumoural TAMs as being significantly higher in the recurrence group of supraglottic laryngeal carcinoma patients but in contrast [15], the density of intratumoural TAMs was significantly higher in cases that had lymph node metastasis. This almost sounds like a plausible reason to also give for ameloblastoma recurrence, until one considers that in the study by Lin JY et al., they marked for pan-TAMs using CD68 that does not discriminate between TAM1 or TAM2 [15]. Hence, the importances of using markers that differentiate one type of TAM from the other, disregarding topography.
Leek RD et al., in their study alluded to the fact that TAMs are seen in the same area of tumour associated vascular density [16]. We found that when considering topography, only few cases had such overlap of MVD and TAMs in comparable regions. Most of our cases did not show related vessel density in areas of TAMs. This may be because the dominant TAM in these areas was TAM1, which is reported to be anti-angiogenic and anti-tumourigenic [14]. Guzman-Medrano R et al., in a similar study reported that they found a high positive correlation between MVD and TAMs [17], however this was not a statistical correlation based on topographical relationship. Hence, we propose that there is a weak topographical relationship between TAMs and MVD in ameloblastomas, mainly because the TAM1 predominates in regions adjacent to the tumour islands.
Limitation
Tumour infiltration by macrophages due to infection or infective processes could not be verified and this could affect interpretation of the results.
Clinical implication: The location of the TAM’s could be informative on possible recurrence potentials of ameloblastomas.
Future prospects: Prospective immunotherapy of ameloblastomas employing TAM1 is an important future point of odontogenic tumour research.
Conclusion
The relative percentage of TAM1 exceeds TAM2 in peritumoural areas of ameloblastoma, conferring anti-angiogenic and hence anti-tumour activity on the tumour.
AUTHOR’S CONTRIBUTION
AAO, USE and GS were involved in the conception and design of the study. AAO, USE and OA were involved in laboratory procedures and data acquisition. AAO and USE performed analysis and wrote the manuscript. GS and SRA gave approval for the study.