The external ear is exposed to various microbes and is a micro-environment in itself. It harbours various bacteria and the health of the external auditory canal is decided by the interplay of various factors like moisture, pH, cerumen and trauma to skin. AOE is the inflammation of the external auditory canal mostly due to bacterial aetiology that results in acute pain and discomfort in the ear. The first case of AOE was described by Toulmousch in 1838 and later systematically described by Mayer in 1844. It was initially thought to be a fungal infection [1]. Investigations initiated during World War II firmly established bacterial aetiology of otitis externa [2,3]. In 1968 Chandler described various aspects of the disease of external ear and organisms were found to be specific for a geographic location and hence a study that would measure common causative organisms of AOE at a given time and a given place is necessary. Microorganisms can be cultured even from the normal external auditory canal, but their mere presence will not account to any pathology in the ear canal [4,5]. It is also important to find out if the common commensals present in auditory canal can cause disease. The aim of this study was to understand the microbiological flora of the normal ear, compare it with the flora of an ear with AOE and to study their significance in etiology. We tried to highlight the need for culture to understand the microbiologic profile and their antibiotic sensitivity. An attempt was made to understand the effect of factors like role of dominant hand on the disease causation and to evaluate the role of normal canal flora and anaerobes in the aetiology.
Materials and Methods
This is a prospective observational study of 64 cases of AOE treated at the outpatient department of Kasturba Hospital, Manipal Academy of Higher Education, Manipal, India, from December 2013 to June 2015. Statistician advice for sample size was sought, Institutional Ethical Committee clearance was obtained and the study was initiated.
Inclusion criteria: All cases presenting with acute onset ear pain (<48 hours) at the outpatient department of ENT were carefully evaluated and the clinically diagnosed cases of unilateral AOE were selected and consent was taken for the participation in the study.
Exclusion criteria: Patients with bilateral AOE, any middle or inner ear pathology in either of the ears. Cases having congenital malformation in the opposite ear, patients who are already on antibiotics for their ear complaints or any other indication and immunocompromised patients, were excluded from the study.
Based on the symptoms and objective otomicroscopic findings, severity was calculated using scoring charts customized for the study. In our study, we have used a “4 point scoring chart” for calculating the subjective and objective assessment scores.
Subjective Assessment Score (SAS) calculation: SAS was calculated by giving a score of 0-2 each for symptoms like pain(0 for no pain, 1 for pain on touch, 2 for pain always), itching (0 for no itch, 1 for occasional itching in the day, 2 for itching throughout the day), discharge (0 for no discharge, 1 for occasional discharge, 2 for continuous discharge) and hard of hearing (0 for no hard of hearing, 1 for mild and 2 for moderate to severe hearing loss affecting daily activities). The SAS range was from 0-8.
Objective Assessment Score (OAS) calculation: OAS was calculated based on otomicroscopy findings like oedema (0 for no canal obliteration, 1 for subtotal and 2 for total canal obliteration), erythema of canal (0 if absent and 1 if present), debris (0 if absent and 1 if present) and discharge (0 for no discharge, 1 for serous/serosanguineous discharge, 2 for purulent discharge) in the canal and OAS ranged from 0-8.
Cumulative Assessment Score (CAS = SAS+OAS) calculation: CAS was noted down at the time of diagnosis (CASpre), after 48 hours (CAS48) of topical therapy by Ichthammol Glycerine (IG) pack. In patients who did not have much relief with IG pack (with a drop in CAS of less than 4), systemic therapy (empirical oral Amoxicilin - Clavulanate along with topical ciprofloxacin drops) was started and a Cumulative Assessment Score after 7 days (CAS7) was calculated.
IG pack was inserted as an initial topical treatment for the condition. The patients were reviewed after 48 hours for pack removal and a repeat otomicroscopy was done and findings were noted. SAS was calculated based on the subjective change in symptoms; OAS was calculated based on repeat otomicroscopy findings and cumulative assessment score (CAS48) was calculated. In subjects with a change in score less than 4, empirical oral antibiotic therapy (oral Amoxicilin – Clavulanate), antibiotic ear drops and pain killers for symptomatic relief were prescribed and were advised to come on follow up after one week to assess the response. Any change in antibiotics as per culture sensitivity report was communicated to the patient. Cumulative Assessment Score after seven days of antibiotics (CAS7) was calculated again to assess response and compare it to the initial score that were calculated after topical therapy (CAS48).
Specimen collection: A detailed history was taken and thorough clinical examination was done. Otomicroscopy was done and under strict aseptic precautions, one swab for aerobic culture and one swab for anaerobic culture were collected from external auditory canals of both the ears. Swabs were collected using sterile cotton applicators from the external auditory canal of both ears separately. The swabs taken for aerobic culture were marked and transferred in the sterile case of cotton applicators. The swabs taken for anaerobic culture were immediately transferred into a freshly prepared Robertson Cooked Meat (RCM) medium procured from the Department of Microbiology just prior to sample collection. So, a total of four swabs (one swab for aerobic culture and one for anaerobic culture from each ear) from every patient were taken, marked separately and were sent to Department of Microbiology.
Specimen processing: In the Department of Microbiology the swabs for aerobic culture were inoculated in 5% Sheep Blood Agar (SBA) [Table/Fig-1], MacConkey agar [Table/Fig-2], and Sabouraud’s Dextrose Agar (SDA), incubated at 37oC for 24-48 hours and the samples were studied after 48 hours. For anaerobic culture, the samples inoculated in Robertson Cooked Meat (RCM) medium at outpatient department were left unopened and incubated at 37oC for 48 hours [Table/Fig-3]. Then it was sub-cultured on SBA, neomycin blood agar and phenyl ethyl alcohol blood agar and incubated in anaerobic chamber (Don Whitely scientific workstation) for 48 hours. If there was no growth, incubation was continued up to five days before a negative report was given.
Sheep blood agar plate showing beta haemolytic golden colour colonies of Staphylococcus aureus.
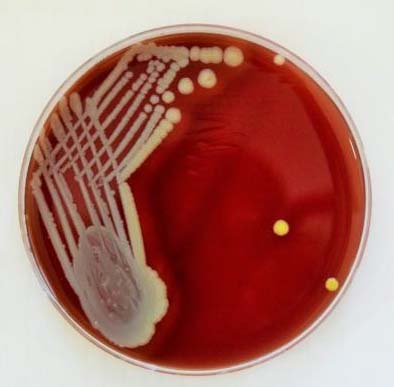
MacConkey agar plate showing colonies of Pseudomonas aeruginosa with metallic sheen.
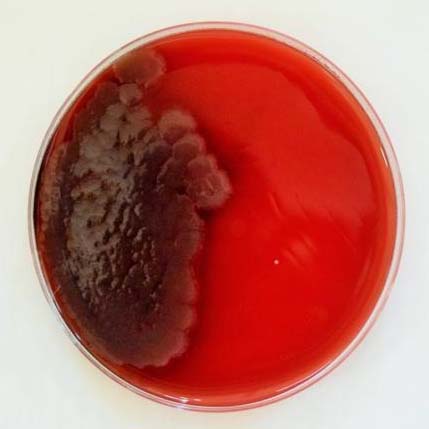
Culture in Robertson Cooked Meat Medium with and without turbidity (turbidity indicates growth of the anaerobe).

Antibiotic susceptibility and aetiology determination: The organisms were identified by colony characters, biochemical reactions as per standard procedures. Later it was confirmed with Matrix assisted LASER desorption ionisation – Time of flight (MALDI TOF). Antibiotic susceptibility testing was done as per Clinical and Laboratory Standards Institute (CLSI) 2004 guidelines [6]. Once an organism was identified, various antibiotic panels were used to study the antibiotic sensitivity as per CLSI guidelines by Modified Kirby Bauer disc diffusion method [6]. For Gram-positive organisms the panel included amoxicillin, oxacillin, cotrimoxazole, amikacin, gentamycin, ciprofloxacin, cephalexin, cefuroxime, vancomycin and linezolid in case of resistant strains. For Gram-negative strains the panel included amoxicillin – clavulanate, ampicillin, ceftriaxone, cefepime, cotrimoxazole, amikacin, gentamycin, ciprofloxacin and netilimycin. In case of resistant strains it was tested for combination antibiotics like piperacillin+tazobactam, cefoperazone+sulbactam, ticarcillin+clavulanate, meropenem. ceftazidime+piperacillin were tested in addition for Pseudomonas aeruginosa. None of the patients in our study had any adverse drug reaction.
Statistical Analysis
SPSS 16.0 software was used for the statistical analysis and data was analysed using Paired t-tests, Wilcoxon signed ranks test and Chi-square test. A p-value of less than 0.05 was considered statistically significant.
Results
In a total of 64 patients included in the study, 34 patients were males and 30 were females. The youngest patient was 10 years (male) and oldest was 66 years (male).The mean age group was 32 years. Incidence was more in the second decade of life.
AOE in right ear was seen in 38 (59.37%) and left ear in 26 (40.62%) subjects. The relation between handedness of the patient and the side on which the subjects commonly developed AOE due to habitual ear fingering was considered. It was found that out of 54 right handed subjects, 36 developed disease in right ear and among 10 left handed persons eight developed disease in left ear. The above numbers indicated that the dominant hand might have a role in causation of the disease but statistically, the p-value was 0.074 (> 0.05) and considered as not significant.
The most common symptom at the time of presentation was ear pain that was seen in 62 subjects. Forty subjects had isolated ear pain and 24 subjects had pain with associated symptoms. The symptoms and signs were found to be more severe in individuals infected with MRSA and scores (SAS and OAS) were > 5 in at least 90% of them. It was also noted that symptoms and signs were severe when the individual had an anaerobic organism growing in the culture as indicated by high CASpre score. There were four individuals who grew anaerobes (two subjects with Clostridium along with Methicillin Sensitive Staphylococcus aureus (MSSA), two subjects with Finegoldia). The CASpre score was about 11 in them which warranted a course of systemic antibiotics in all four of them.
In 30 subjects, the score after 48 hours of IG pack (CAS48) did not show a decrease of more than four when compared to the score before the treatment was initiated (CASpre), hence systemic antibiotic therapy was started and eight subjects among these, showed symptoms even after a week of systemic therapy with a decrease in CAS7 less than four and culture directed therapy was needed to relieve the symptoms.
A single organism (either aerobic/anaerobic/fungus) was cultured in 50 subjects and more than one organism (polymicrobial growth) was seen in 10 subjects (13%) and no growth was seen in cultures of four subjects in the study [Table/Fig-4].
various organisms isolated from the ear swabs collected from test ears.
| Organism isolated (aerobes) | No. of subjects | Co-existent organisms (number of subjects) |
|---|
| Pseudomonas aeruginosa | 22 | Proteus spp.(2), E.coli (2) |
| MRSA‡ | 12 | - |
| MSSA† | 8 | Acenitobacter (2),*Clostridium (2) |
| CoNS§ | 8 | Corynebacterium striatum (2) |
| Aspergillus | 4 | - |
| Klebsiella | 2 | - |
| Morganella | 2 | - |
| No aerobe growth | 4 | - |
| *Finegoldia magna | 2 | |
anaerobes,
MRSA – Methicillin Resistant Staphylococcus aureus,
MSSA – Methicillin Sensitive Staphylococcus aureus,
CoNS – Coagulase Negative Staphylococcus
In this study, there was no co-existent organism with MRSA. It was noted that Staphylococcus was the most common organism at a genus level as it includes MRSA, MSSA and Coagulase Negative Staphylococci (CoNS) isolated in 28 subjects. At a species level, Pseudomonas aeruginosa was the most common species isolated in 22 subjects [Table/Fig-5].
Culture sensitivity and resistance pattern of the organisms isolated from test ear swabs.
| Organism isolated (no. of subjects) Main pathogen | Coexistent pathogen (no of subjects) | Sensitive to | Resistant to |
|---|
| MRSA (12) | Nil | Amikacin, cotrimoxazole, doxy/tetracycline | Amox-clav, ampicillin, ciprofloxacin |
| Pseudomonas aeruginosa (22) | Nil (18) | Amikacin, cotrimoxazole, doxy/tetracycline, gentamycin | In one case - ceftazidime, ciprofloxacin, gentamycin |
| With Proteus (2) | Amikacin, cotrimoxazole, doxy/tetracycline, gentamycin | |
| With E. coli (2) | Amikacin, gentamycin | Ciprofloxacin, amox-clav |
| MSSA(8) | Nil(4) | Cotrimoxazole, doxy, amox-clav (1) | Amox-clav (1), ampicillin, ciprofloxacin |
| With Acinetobacter (2) | Ciprofloxacin, cotrimoxazole, gentamycin | Amox-clav, ampicillin, ciprofloxacin |
| With Clostridium (2) (Anaeobe) | Cotrimoxazole, gentamycin, doxy/tetracyclin | Amox-clav, ampicillin, ciprofloxacin |
| CoNS(8) | | - | - |
| Aspergillus (4) | | - | - |
| Klebsiella (2) | | Amox-clav, ciprofloxacin, amikacin | Ampicillin, amoxycilin |
| Morganella (2) | | Amikacin, ceftriaxone, cotrimoxazole | Amox-clav, ampicillin, ciprofloxacin |
| Finegoldia magna (2) (Gram-Positive Anaerobic Cocci) | | Metronidazole | |
It was noted that all subjects who grew MRSA, MSSA, Morganella and Pseudomonas (with co-existent E. coli) were resistant to the routine antibiotic treatment given i.e., oral amoxycillin - clavulanate and topical ciprofloxacin drops. These bacteria were more sensitive to cotrimoxazole when compared Amox-clav. Cotrimoxazole is not preferred most of the times for the fear of Stevens-Johnson syndrome. Pseudomonas was found to be sensitive to aminoglycosides. CoNS were considered as normal skin commensals, so sensitivity was not done. Aspergillus spp. was isolated in four subjects and was effectively treated with IG pack and needed no further treatment. Antibiotic sensitivity for fungus was not done in our study as they required specific panels. Subjects with anaerobes, Finegoldia magna (2) and Clostridium isolated along with MSSA (2) had more severe symptoms and had to be started on systemic antibiotics as the IG pack did not give much relief with a decrease in CAS48 <4. As the anaerobic organisms are known to grow deeper in the tissues, it warranted a course of systemic antibiotics for these four subjects.
The swabs from the control ear showed growth in ten subjects. Nine subjects were found to grow coagulase negative Staphylococcus (two of the nine subjects had Staphylococcus epidermidis) and one subject was found to grow Klebsiella pneumoniae, which was found to be sensitive to amox-clav, ciprofloxacin, amikacin and resistant to ampicillin and amoxicilin.
Discussion
AOE is an inflammatory (typically infectious) disorder of the external ear canal [7]. AOE is a generalized condition of the skin of the external auditory canal that is characterized by oedema and erythema of canal skin associated with itchy discomfort and usually an ear discharge [8].
In present study, incidence of AOE was more during the second decade of life. People in this age group start going outdoors to play and actively participate in school and college activities exposing the canal to various bacteria and may end up in AOE when conditions favour the growth of those organisms in the canal. There is no marked sex predilection in the incidence of AOE noted in our study and the fact that males have more exposure to outdoors compared to females in Indian population would have resulted in a slight increase in male subjects in this study.
Pseudomonas aeruginosa and Staphylococcus aureus are the most common causative bacteria for AOE however, it may be due to other bacteria as well [4,9]. It may also be due to fungal/viral aetiology as well. The normal commensals like Staphylococcus epidermidis and Corynebacterium may be present in the cultures. Considering the fact that people would reach out for the ear on their dominant side in case of habitual ear picking causing finger nail trauma to the canal an effort was made to assess the statistical significance of dominant hand in causing AOE in the ear of that side. Although the figures suggested that there might be a correlation, but the association was statistically non-significant (p= 0.074).
The normal External Auditory Canal (EAC) also has microbiological flora, 90% of which would be Gram-positive bacteria living as commensals in the canal. The normal flora may turn pathogenic when the protective barriers like cerumen, canal pH, diabetes and other immune compromised states fail to protect the ear canal [10]. According to study by Perry ET et al., Micrococcus spp. were found in normal ear canals of 90 healthy individuals and stated trauma to canal skin or increased environmental heat or humidity to be the causative factors for the disease [10]. Goguen LA et al., in their study have noted that staphylococci were the most common (63%) bacteria in normal ear canal (Staphylococcus auricularis and Staphylococcus epidermidis), Pseudomonas aeruginosa was the most common cause of AOE and anaerobic organisms caused AOE in 4%-25% of the patients [11] which is similar to present study, where P. aeruginosa was the most common species isolated. Staphylococcus aureus and Pseudomonas aeruginosa were found to be to be the most common organisms causing AOE in a prospective study done by Clark WB et al., [12]. In another study, Gram-negative organisms were more frequently isolated than Gram-positive organisms from the subjects [4].
Lab testing and cultures are helpful in treating severe disease and in recurrent cases. Anaerobic cultures are desirable if not mandatory in studying the aetiology of the disease [6]. Cultures are advisable in patients with deranged immune status like post transplant status, HIV, patients on chemoradiation and also in patients with history of ear surgeries or in patients not responding to initial therapy [13]. Imaging studies are helpful if an invasive infection such as necrotising (malignant) AOE is suspected or if the diagnosis of mastoiditis is being considered. Imaging modalities may include the following: High resolution computed tomography (HRCT) - preferred as it depicts bony erosions, Radionucleotide bone scanning, Galium scanning, Magnetic resonance imaging (MRI) - considered if soft tissue extension is the predominant concern [14].
The American Academy of Otolaryngology – Head and Neck Surgery Foundation (AAO-HNSF) developed a Clinical Practice Guideline in 2006 for the treatment of AOE [15]. The group recommends use “topical preparations for initial therapy of diffuse, uncomplicated AOE; systemic antimicrobial therapy should be used if there is extension outside of the ear canal or the presence of risk factors like diabetes, prior radiotherapy, or immune compromise. In present study, IG pack was inserted and used as initial therapy to relieve itching and other symptoms related to AOE. Ichthammol is ammonium bituminosulfonate, which has anti-inflammatory, bactericidal, and fungicidal properties. Roland PS et al., performed one of the few randomized multicenter studies comparing ototopical antibiotics in which ciprofloxacin/dexamethasone showed higher bacterial eradication rates and more rapid symptom improvement compared with neomycin/polymyxin B/hydrocortisone [16]. In December 2014, the FDA approved the fluoroquinolone antimicrobial finafloxacin otic suspension for the treatment of AOE caused by Pseudomonasaeruginosa and Staphylococcus aureus [17]. In present study, if the IG topical did not improved the CAS score, empirical antibiotics with topical ciprofloxacin (a fluoroquinolone) were prescribed to the patients. Nowadays, AOE is a common condition being treated in everyday otology practice and thorough knowledge of the disease and the pathogens common in the particular geographical area and population would help in treating this disease better.
Limitation
The possible limitation of the study is the fact that scoring was done based on subject’s personal perception of improvement in symptoms - SAS (ranging from 0-8). Also, due to limited resources, antibiotic sensitivity was not done for fungal isolates in present study.
Conclusion
Whenever the standard topical medication is not helping to alleviate the symptoms of AOE, tackling the predisposing factors and a culture directed treatment would be very effective in treating AOE so that spread of resistance among pathogens can be prevented.
*anaerobes,
‡MRSA – Methicillin Resistant Staphylococcus aureus,
†MSSA – Methicillin Sensitive Staphylococcus aureus,
§CoNS – Coagulase Negative Staphylococcus