Case Report
A 32-year-old, G2P1L1 female with 31 weeks of gestation presented in Obstetric Outpatient Department with a positive IAT reported from outside laboratory. The patient was Rh negative & thus suspected of Rh isoimmunisation. However, patient had past history of receiving single dose of anti D prophylaxis, 4000U during first pregnancy. Previous pregnancy led to full term normal delivery of healthy child with no history of neonatal anemia or jaundice. There was no significant medical history or obstetric history of any still births, abortions or medical termination of pregnancy. There was no past history of blood transfusion. Patient had not received anti D in this pregnancy so far.
In view of positive IAT, Doppler ultrasound was done which revealed Middle Cerebral Artery Peak Systolic Velocity (MCA-PSV) > 1.5 Multiples of Median (MOM); suggestive of severe fetal anemia. The patient was planned for Intrauterine Transfusion (IUT), however multiple units of O Rh negative leukoreduced packed RBCs put up for cross-match were found to be incompatible with maternal serum. Hence, the patient was referred to our Regional Blood Transfusion Centre (RBTC) for immunohaematology work-up.
First of all, ABO blood grouping and D typing of patient and her husband were performed. Patient’s forward and reverse blood grouping done at room temperature (22°C) showed discrepancy. Following which, the patient’s blood sample was collected in EDTA vial under strict warm conditions. The RBCs and serum were separated immediately by centrifugation at 2000 rpm for 5 minutes. The cells were washed multiple times with warm normal saline. Extended forward and reverse blood grouping was done at 22°C, 37°C and 4°C by tube method. The blood group was confirmed as AB negative at 4°C. [Table/Fig-1] Patient was further confirmed to be negative for weak D by IAT using tube method and subsequently by column agglutination technology (Diamed gel card method, Diamed, Switzerland). Autocontrols were negative at 3 temperatures ruling out autoantibody. Husband’s blood group was A Rh positive.
Extended Blood grouping results of the patient.
| Temperature(°C) | >Forward Grouping | Reverse Grouping |
|---|
| Anti A | Anti B | Anti D 1 | Anti D 2 | NS* | BG† | A cells | B cells | O cells | AC‡ | BG |
|---|
| 4 | 4+ | 4+ | Neg§ | Neg | Neg | AB Neg | Neg | Neg | Neg | Neg | AB Neg |
| 22 | 4+ | 4+ | Neg | Neg | Neg | AB Neg | 4+ | 4+ | 4+ | Neg | In-valid |
| 37 | 3+ | 4+ | Neg | Neg | Neg | AB Neg | 2+ | 2+ | 2+ | Neg | In-valid |
*NS- normal saline, †BG- blood group, ‡AC- autocontrol, §Neg- Negative
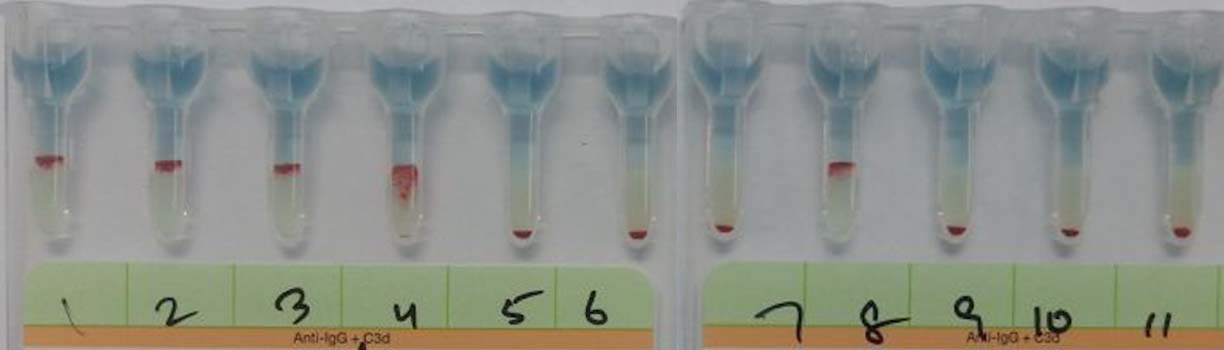
Also, polyspecific Direct Antiglobulin Test (DAT- anti IgG and C3d) of the patient was negative. A commercially available three-cell antigen panel (ID-DiaCell I-II-III Asia, Diamed, Switzerland) was used for antibody screening by IAT. The patient’s serum was reacted with reagent RBCs using LISS/Coombs ID-cards, at 37°C in AHG (anti human globulin) phase. The cards were incubated for 15 minutes and then centrifuged in ID-centrifuge for 10 minutes. The antibody screening panel was positive showing pan-agglutination. However, IAT by 3 cell panel was negative at 4°C in saline phase. An extended 11-cell panel ID-DiaPanel, DiaMed [Table/Fig-2] was used for antibody identification by IAT using ID-cards at 37°C. The reactions were suggestive of anti C + anti D antibodies [Table/Fig-3].
Phenotype of reagent red cells of extended 11-cell panel (DiaMed 11 cell ID-DiaPanel) used for antibody identification by IAT.
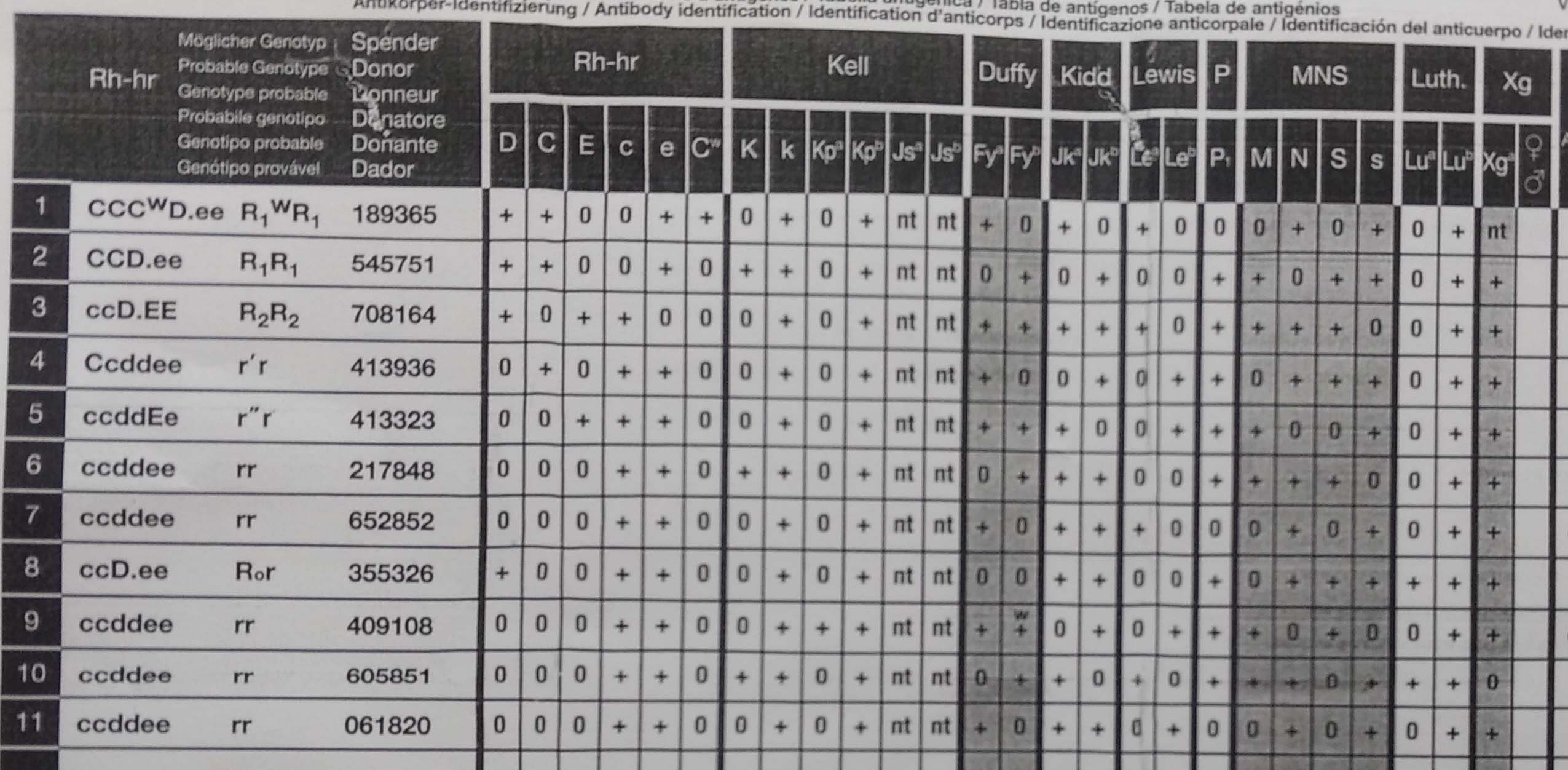
Indirect antiglobulin test using 11-cell panel showing a reactivity pattern suggestive of anti C + anti D antibodies.
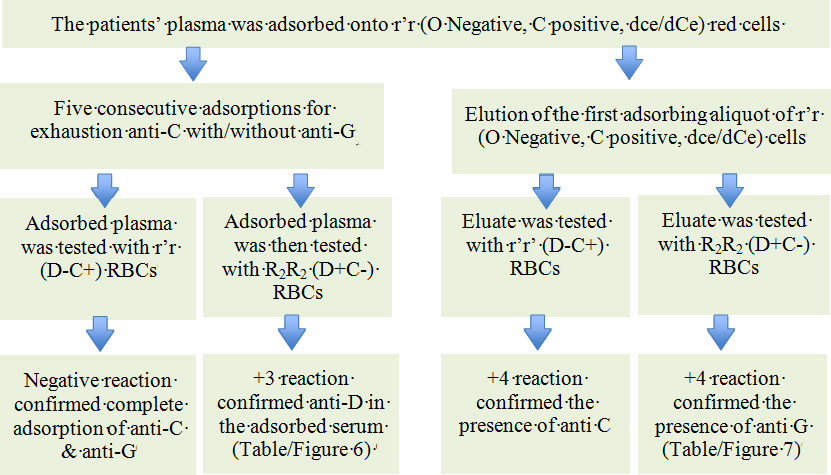
Rh/Kell/extended antigen profile of patient and her husband was done by using column agglutination technology (DiaClon gel card, Diamed Switzerland). Husband was strongly positive for C antigen whereas the patient was negative for it. [Table/Fig-4] Therefore, possibility of anti D + anti C with or without anti G could not be excluded. Meanwhile, a unit of irradiated, O Rh negative, C negative leucoreduced packed RBCs (haematocrit of 80%) was found to be compatible with maternal serum and was successfully transfused in-utero. Pre-transfusion foetal haemoglobin was 3.3 g/dl, blood group B negative and DAT strongly positive. Fetal blood was not available for Rh/Kell/extended antigen profile. In view of Rh negative foetus, possibility of Hemolytic Disease of Foetal and Newborn (HDFN) due to anti C + anti G was considered. Patient’s plasma was further tested by differential adsorption and elution using r’r (O Negative, C positive, dce/dCe) RBCs [Table/Fig-5]. Elution was done using DiaCidel acid elution kit, Diamed, Switzerland. The same process was repeated using R2R2 (AB positive, C negative, DcE/DcE) RBCs for confirmation. A final diagnosis of Anti D + C + G alloantibodies was made [Table/Fig-6,7]. Titres were done by using D+C- RBCs for anti D (1:64) and using D-C+ RBCs for anti C (1:4) antibody by tube method at 37°C. Due to rarity of rGr cells, a direct evidence and titre of anti G could not be established.
Rh/Kell/extended antigen profile of patient and her husband.
| Antigen | C | c | E | e | K | control |
| Patient | Neg | 4+ | Neg | 4+ | Neg | Neg |
| Husband | 4+ | Neg | Neg | 4+ | Neg | Neg |
*Neg- negative
Flow chart of differential adsorption and elution for differentiation and identification of anti D, anti C and anti G.
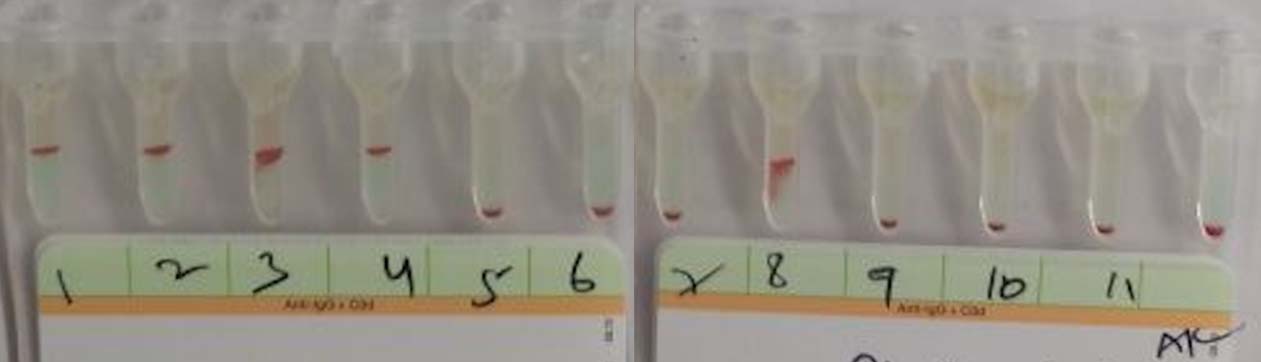
Results of IAT with plasma adsorbed using r’r (O Negative, C positive, dce/dCe) RBCs. Reactions with 1st to 3rd cells and 8th cell confirmed the presence of anti D.
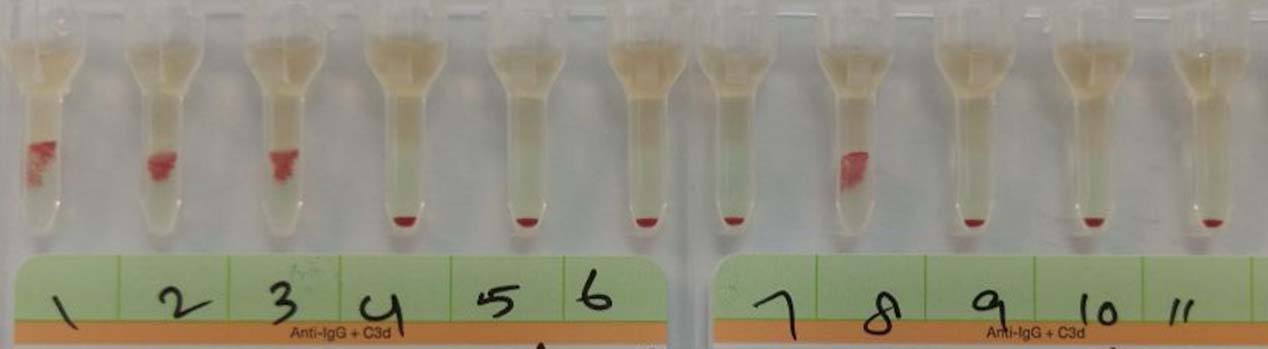
Results of IAT with eluate prepared from the first adsorbing aliquot of r’r (O Negative, C positive, dce/dCe) cells. Reactions with 1st, 2nd and 4th cells confirmed anti C while reactions with 3rd and 8th cells suggested anti G.
At 34 weeks of gestation, patient underwent spontaneous preterm delivery of low birth weight baby. The newborn had severe pallor and mild icterus (Hb 4.5 g/dl, serum bilirubin 4 mg/dl, post-IUT DAT trace +, eluate - negative). The neonate was kept in phototherapy. However, serum indirect bilirubin rapidly increased to 17 mg/dl. The neonate underwent Double Volume Exchange Transfusion (DVET) with O Rh (D) negative C, c, E and Kell negative fresh whole blood compatible with maternal and foetal serum. However, the baby developed sepsis and died of multiple organ failure on day 4.
Discussion
Rh blood group system is a complex system with about 50 different antigens including D, c, E, C and e in order of immunogenicity. Two closely linked genes (RHD and RHCE genes) on chromosome 1 control the expression of Rh antigens [1]. The “Rhesus G” antigen was first described by Allen FH Jr and Tippet PA in 1958 [2]. It is encoded within the C encoding gene [3]. The erythrocytes bearing C and/or D antigen exhibit the G antigen and its expression is maximum when both D and C antigens are present. The RBCs that do not express both C and D antigen are usually negative for G antigen [4]. This co-distribution of G with either C or D antigen gives anti G antibody the apparent anti C + anti D specificity [5].
Therefore, alloimmunized pregnant women showing a reactivity pattern of anti C + anti D, should be worked-up for underlying anti G as it may mimic anti C/D and cause HDFN either in combination or independently [6-11].
Pahuja S et al., screened 3,577 multigravida women for the presence of alloantibodies. An overall alloimmunisation rate of 1.25% was reported. Among these, the majority accounted for anti D (78.43%) and anti C + anti D (11.76%) was the most common combination [12]. Similarly, Nordvall M et al., found anti D + anti C was the most common combination (52, i.e., 43%) in their study of 122 cases with multiple antibodies [13].
Since G antigen is present on all RBCs that are C+ and also on most (not all) D+ RBCs [14]. The differentiation of anti D, C, and G specificities in alloimmunized pregnancies is essential for clinical prognosis as well as to decide whether Rh(D) prophylaxis should be given or not [7].
Also this complex serological finding often accounts for: 1) Diagnostic dilemmas like anti D like picture in the maternal serum despite Rh D prophylaxis during previous pregnancy; 2) Social complications like HDFN due to anti D when the father is D negative; or 3) Medicolegal implications like anti D like picture in an Rh (D) negative patient transfused with Rh negative blood. The antibodies in such cases could actually be anti G + C [15].
Baia F et al., studied 32 serum samples showing an apparent anti D+C specificity and found anti D+C+G (n=10, 37.04%) to be the most common combination followed by anti C+G (n=10, 37.04%), anti D+C (n=5, 18.5%), anti D+G (n=5, 18.5%) and anti G alone (n=2, 7.41%) [16]. Palfi M et al., analyzed sera from 27 alloimmunized women with anti D+C, by adsorption/elution studies. The combination of D+C+G, D+G, G+C and D+C antibodies occurred in 48.1%, 25.9%, 14.8% and 11.1% of the women, respectively. Overall, anti G was spotted in 24/27 samples (88.9%) [10]. Hence, anti G antibody may not be so rare and perhaps it is often under-diagnosed due to lack of awareness or limited resources. To the best of our knowledge there is only a single case of anti D+C+G reported from India so far, by Makroo RN et al., [15].
The G antigen in association with D antigen is moderately immunogenic, eliciting anti G in approximately 30% of D and G-negative individuals [5]. However, the production of anti G by D-negative but C and G positive cells is exceedingly rare [17]. Therefore, considering G antigen for routine transfusion in Rh negative patients is not necessary. For Intra-Uterine Transfusion (IUT) in HDFN, cross-matched Cytomegalovirus seronegative, irradiated group O Rh(D) negative, C negative and consequently G negative RBCs would be indicated. Maternal RBCs can also be used for IUT [14].
Paternal antigen profile should always be done. If the father is negative for antigen corresponding to maternal alloantibody, no further intervention is needed for foetus. However if the father is positive, the paternal genotype should be analysed for homo-/hetero-zygosity to determine the risk of HDFN [18].
A titer of antibody against D or another paternal antigen > 1:16 in albumin or 1:32 by IAT necessitates Doppler study of MCA-PSV for antenatal monitoring [19]. The Scientific Section Coordinating Committee of the AABB states that a rising titer or critical titer of 16 by saline tube antiglobulin procedure indicates the need for amniotic fluid analysis. It recommends the use of r’r and R2R2 RBCs for titration to avoid inter-laboratory variation due to phenotypic variation in reagent cells [20].
The role of anti G in causing HDN is debatable [6-11]. According to Palfi M et al., anti G rarely presents with a high titer that may affect the foetus. In their study, none of the four children born to women with anti G + anti C required medical intervention [10]. In contrast, another study found two cases with anti G titers consistent with moderate to severe HDFN by chemiluminescence. It was also noted that HDN due to anti G was more likely to occur in r’r (C-positive, D-negative) fetuses [8]. Trevett TN Jr and Moise KJ Jr reported a case of twin pregnancy with severe HDN due to anti G + C similar to our case [21].
Conclusion
The aim is to highlight that non-anti D antibody should be considered in alloimmunized women who give a suggestive history but no beneficial effect of anti D prophylaxis. The differentiation of anti D, C, and G is vital from clinico-therapeutic, prognostic, social and medico-legal perspective. The present case is being reported owing to the rarity of HDFN caused by anti C + anti G antibody. Parent’s antigen profile and antibody identification also helped to find suitable antigen negative blood units for IUT and Double Volume Exchange (DVET).
*NS- normal saline, †BG- blood group, ‡AC- autocontrol, §Neg- Negative
*Neg- negative
[1]. Negi G, Singh GD, Anti Rh Hemolytic Disease due to Anti C Antibody: Is Testing for Anti D Antibodies Enough?Indian Journal of Haematology & Blood Transfusion 2012 28(2):121-22. [Google Scholar]
[2]. Allen FH, Jr, Tippett PA, A new Rh blood type which reveals the Rh antigen GVox Sang 1958 3:321-30. [Google Scholar]
[3]. Faas BH, Beckers EA, Simsek S, Overbeeke MA, Pepper R, van Rhenen DJ, Involvement of Ser103 of the Rh polypeptides in G epitope formationTransfusion 1996 36:506-11. [Google Scholar]
[4]. Skov F, Observations of the number of available G (rhG, Rh12) antigen sites on red cellsVox Sang 1976 31:124-30. [Google Scholar]
[5]. Mollison PL, Blood transfusion in clinical medicine 1983 7th editionBlackwell Scientific Publications:340 [Google Scholar]
[6]. Schulze TJ, Goebel M, Scharberg EA, Bugert P, Janetzko K, Development of anti-G, anti-C and anti-Jk(b) in a 22-year-old mother during her fourth pregnancyTransfus Med Hemother 2013 40:207-09. [Google Scholar]
[7]. Yousuf R, Mustafa AN, Ho SL, Tang YL, Leong CF, Anti-G with concomitant anti-C and anti-D: A case report in a pregnant womanAsian J Transfus Sci 2017 11:62-64. [Google Scholar]
[8]. Hadley AG, Poole GD, Poole J, Anderson NA, Robson M, Haemolytic disease of the newborn due to anti-GVox Sang 1996 71:108-12. [Google Scholar]
[9]. Yesus YW, Akhter JE, Hemolytic disease of the newborn due to anti-C and anti-G masquerading as anti-DAm J Clin Pathol 1985 84:769-72. [Google Scholar]
[10]. Palfi M, Gunnarsson C, The frequency of anti-C + anti-G in the absence of anti-D in alloimmunized pregnanciesTransfus Med 2001 11:207-10. [Google Scholar]
[11]. Huber AR, Leonard GT, Driggers RW, Learn SB, Gilstad CW, Case report: Moderate hemolytic disease of the newborn due to anti-GImmunohaematology 2006 22:166-70. [Google Scholar]
[12]. Pahuja S, Gupta SK, Pujani M, Jain M, The prevalence of irregular erythrocyte antibodies among antenatal women in DelhiBlood Transfusion 2011 9:388-93. [Google Scholar]
[13]. Nordvall M, Dziegiel M, Hegaard HK, Bidstrup M, Jonsbo F, Christensen B, Red blood cell antibodies in pregnancy and their clinical consequences: synergistic effects of multiple specificitiesTransfusion 2009 49:2070-75. [Google Scholar]
[14]. Rikabi N, Dunn-Albanasse L, Krugh D, Snider D, Frenken K, Rossi K, Is It Really Anti-D and Anti-C or Is It Anti-G?Laboratory Medicine 2003 34(3):193-96. [Google Scholar]
[15]. Makroo RN, Kaul A, Bhatia A, Agrawal S, Singh C, Karna P, Anti-G antibody in alloimmunized pregnant women: Report of two casesAsian Journal of Transfusion Science 2015 9(2):210-12. [Google Scholar]
[16]. Baía F, Muñiz-Diaz E, Boto N, Salgado M, Montero R, Ventura T, A simple approach to confirm the presence of anti-D in sera with presumed anti-D+C specificityBlood Transfusion 2013 11(3):449 [Google Scholar]
[17]. Jakobowicz R, Whittingham S, Barrie JU, Simmons RT. A further investigation on polyvalent anti-C (rh’) and anti-G (rho) antibodies produced by iso-immunization in pregnancyMed J Aust 1962 1:896-97. [Google Scholar]
[18]. Gajjar K, Spencer C, Diagnosis and management of non-anti-D red cell antibodies in pregnancyThe Obstetrician and Gynaecologist 2009 11(2):89-95. [Google Scholar]
[19]. Suresh B, Deepthi K, Yashovardhan A, Arun R, Babu KS, Jothibai DS, Haemolytic disease of the newborn due to multiple maternal antibodiesJ Clin Sci Res 2014 3:198-201. [Google Scholar]
[20]. Judd WJ, Practice guidelines for prenatal and perinatal immunohaematology, revisitedTrans- fusion 2001 41:1445-52. [Google Scholar]
[21]. Trevett TN Jr, Moise KJ Jr, Twin pregnancy complicated by severe hemolytic disease of the fetus and newborn due to anti-g and anti-CObstet Gynecol 2005 106(5 Pt 2):1178-80. [Google Scholar]