An Alpha Fetoprotein Producing Gastric Tumor with Yolk Sac, Hepatoid and Papillary Adenocarcinoma Components
Archana Lakshmanan1, Ann Kurian2, Annapurneswari Subramanyan3, Ayyappan Srinivasan4
1 Junior Consultant, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
2 Senior Consultant, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
3 Senior Consultant, Department of Histopathology, Apollo Hospitals, Chennai, Tamil Nadu, India.
4 Senior Consultant, Department of Surgical Oncology, Apollo Hospitals, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Archana Lakshmanan, New No.6, Old No.24, Cenotaph Road, Teynampet, Chennai-600035, Tamil Nadu, India.
E-mail: mrithulaarchana@gmail.com
Alpha Fetoprotein (AFP) producing gastric carcinomas are very rare and have unique clinicopathological features and an extremely poor prognosis. Here, we report a case of AFP producing gastric carcinoma with three distinct histomorphologic patterns such as yolk sac like, hepatoid, tubular and papillary adenocarcinoma components. The uniqueness of this case is absence of metastases and associated findings such as fundic gland polyposis with varying degrees of dysplasia, gastric and duodenal well differentiated neuroendocrine tumour and rectal ganglioneuroma. The patient is symptom free as of two and half year’s postoperative period. AFP producing gastric tumours although rare, need to be identified as it is known to carry poor prognosis.
Gastric carcinoma, Stomach, Tumour marker
Case Report
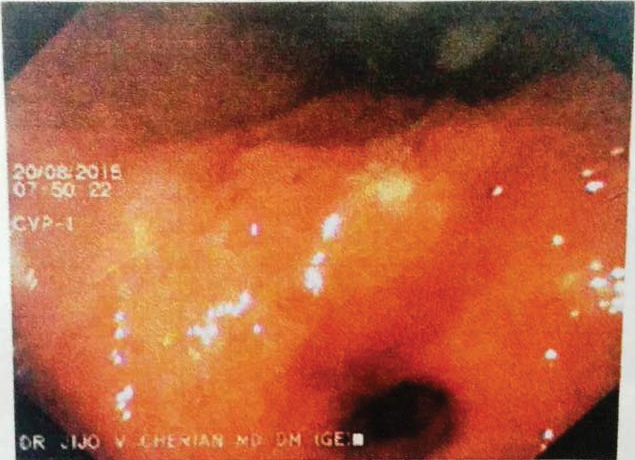
A 75-year-old male presented with history of abdominal pain and generalised weakness for about three months. Clinically he was anaemic and per abdomen examination was unremarkable. Oesophagogastroduodenoscopy revealed an ulceroproliferative polypoidal growth involving the gastric antrum [Table/Fig-1] and few scattered small sessile nodules in the body and antrum of stomach. Colonoscopy revealed a polyp in the rectum. The gastric antral lesion was biopsied and rectal polyp was excised and sent for histopathological examination. The patient also had an enlarged left supraclavicular lymph node which was also biopsied. Gastric antral lesion was reported as a moderately differentiated adenocarcinoma with papillary pattern and the rectal polyp was reported as ganglioneuroma [Table/Fig-2]. The supraclavicular lymphnode showed a necrotising granulomatous inflammation, however the special stains such as Zeihl- Neelsen and Periodic Acid Schiff (PAS) for tuberculosis and fungi were negative.
Oesophagogastroduodenoscopy shows a raised nodular lesion in the gastric antrum.
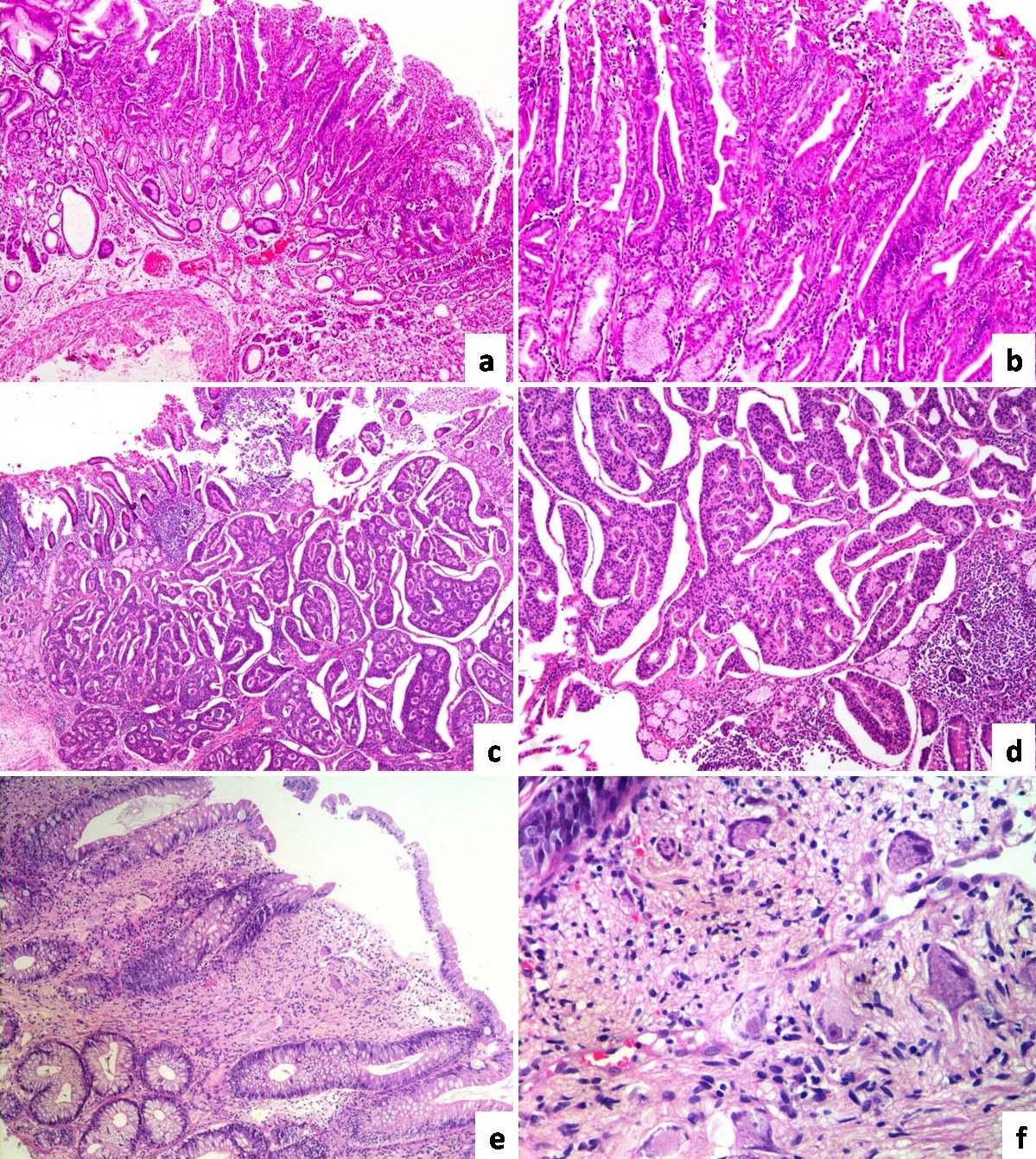
Low and high power view of Fundic gland polyp exhibiting mild dysplasia: (a) (H and E 4X); and (b) (H and E 10X); Well differentiated neuroendocrine tumour involving duodenum: (c) (H and E 10X); and (d) (H and E 40X); Ganglioneuroma involving the rectum: (e) (H and E 10X); and (f) (H and E 40X).
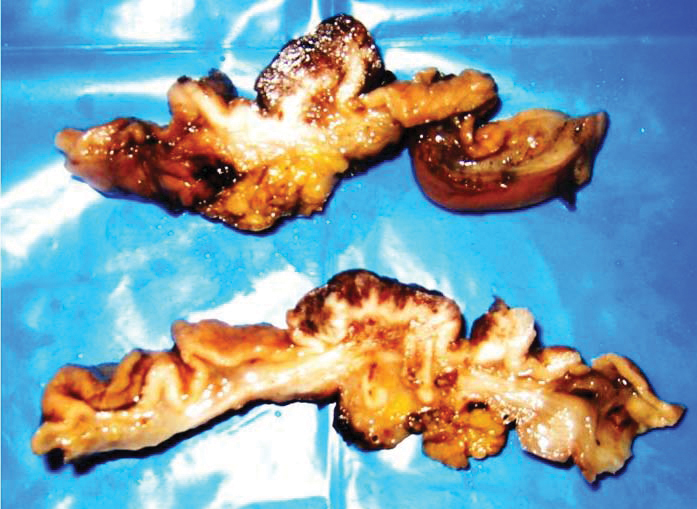
A radical subtotal gastrectomy with D2 lymphadenectomy and Billroth I anastomosis with feeding jejunostomy was done. Specimen was fixed in 10% neutral buffered formalin and representative sections were taken. Grossly, there was a raised ulcerated lesion involving the gastric antrum along the greater curvature measuring 5.0 x 3.0 x 1.9 cm [Table/Fig-3]. Cut surface of the lesion was grey white, firm and involved the gastric wall transmurally extending focally into omental adipose tissue. There were few small sessile polypoidal projections seen scattered in the rest of the gastric mucosa ranging in size from 0.3 to 0.5 cm. Few enlarged perigastric lymph nodes were noted, which were also sampled. The sections were then processed, paraffin embedded, 4 micron thin sections were cut and stained with Haematoxylin-Eosin (H and E), PAS and Periodic Acid Schiff with Diastase (PASD). Immunohistochemical staining was performed in Ventanna automated immunostainer (Benchmark XT) using manufacturer’s protocol.
Gross image of the tumour seen involving the entire thickness of gastric wall.
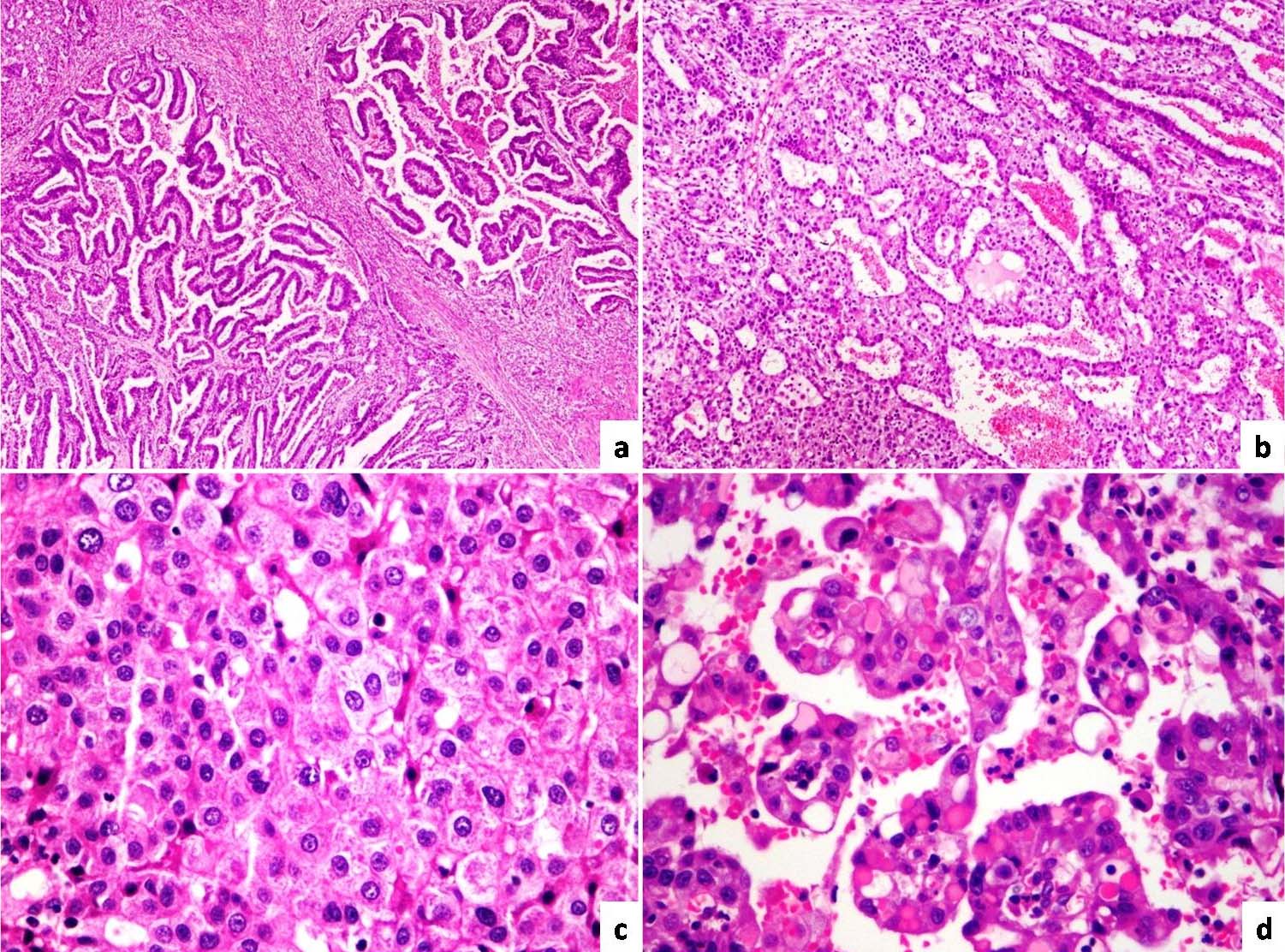
Microscopically there was a malignant tumour with three distinct histomorphologic patterns [Table/Fig-4]. The predominant pattern was yolk sac like areas with characteristic reticulated and microcystic pattern lined by single layer of low cuboidal cells. The second common pattern was tubular and papillary adenocarcinoma with tubules and papillae lined by stratified columnar epithelium exhibiting moderate nuclear atypia. Amidst these two patterns, there are sheets and trabeculae of large polygonal cells with abundant pale eosinophilic cytoplasm, vesicular nuclei with prominent nucleoli morphologically resembling hepatocellular carcinoma. Few intracytoplasmic hyaline globules which are PAS positive and Diastase resistant were noted within yolk sac like and hepatoid areas. There was no evidence of lymphovascular invasion. Perigastric lymphnodes showed reactive features.
Papillary adenocarcinoma component: (a) (H and E 4X); (b) (H and E 10X) Yolk sac component; (c) Hepatoid areas (H and E 40X); (d) Hyaline globules (H and E 40X).
Histopathological examination of the other sessile polyps revealed fundic gland polyps with varying levels of dysplasia. The sections submitted as proximal resected margin (stomach) and distal resected margin (duodenum) showed a well differentiated neuroendocrine tumour within the lamina propria.
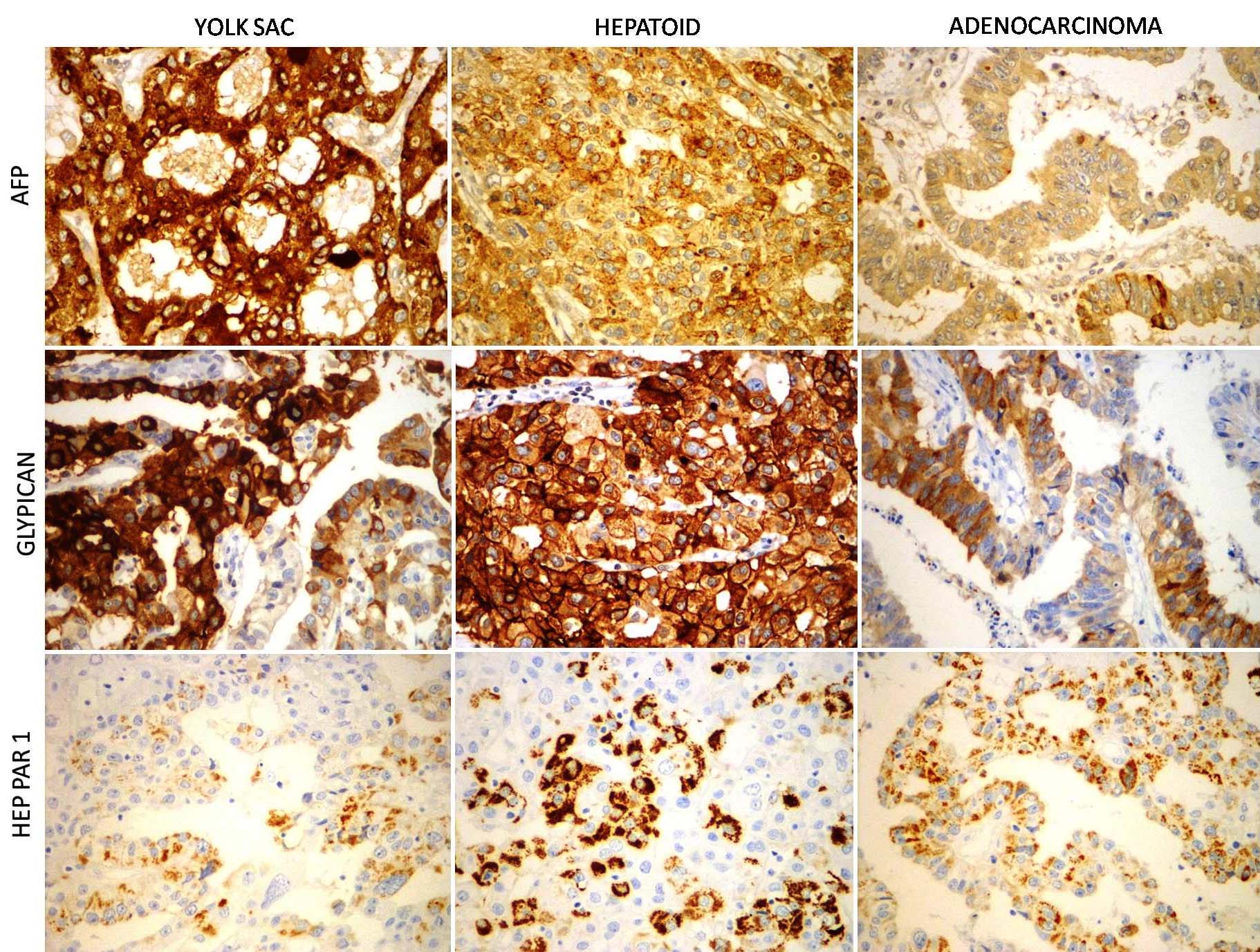
Immunohistochemical findings are as mentioned in [Table/Fig-5]. The gastric antral lesion was diffusely positive for cytokeratin. AFP and glypican were diffusely positive in yolk sac like and hepatoid areas, focally positive in adenocarcinoma areas. Hep-Par1 was focally positive in all three components. Carcinoembryonic Antigen (CEA) was variably positive in all the areas. Immunostains for beta- Human Chorionic Gonadotrophin (HCG), CD30 and Placental Alkaline Phosphatase (PLAP) were negative. The lesion in the proximal and distal resected margins was diffusely positive for synaptophysin, chromogranin and cytokeratin (cytoplasmic, dot like). The proliferation index was very low (Ki67<2%). Hence a diagnosis of AFP gastric tumour with yolk sac, hepatoid and papillary adenocarcinoma components was rendered. The postoperative period was uneventful. Serum AFP was measured after only after complete surgical resection and was found to be elevated (65ng/ml). A 30 months postoperative visit, the patient is symptom free.
AFP and glypican are strongly positive in yolk sac and hepatoid areas and focally positive in papillary adenocarcinoma areas. HEP-PAR1 is variably positive in all the components (IHC 40X).
Discussion
AFP is an oncofetal glycoprotein which is secreted by fetal liver, yolk sac and by some gastrointestinal cells, which rapidly decrease after birth [1]. AFP is used as a serum tumour marker for screening and monitoring yolk sac tumours and hepatocellular carcinoma [2,3]. Elevated serum AFP has also been reported in carcinomas of various other organs [4,5]; of which stomach is the most common site [6-10].
AFP producing gastric cancers is rare, with reported incidence of 1.3% to 15% in varied geographical locations [6-10]. These tumours are associated with aggressive clinical behaviour and pooror prognosis compared to AFP negative gastric cancer. AFP positive gastric cancer has the characteristics of high proliferation index, higher staging, extensive lymphovascular invasion, lymph node metastasis and liver metastases, short survival time and poor prognosis [6,7]. Morphologically these tumours can often have varied histomorphologic patterns, of which hepatoid pattern is the most common [8,9]. The other patterns are yolk sac like, tubulopapillary adenocarcinoma, poorly differentiated medullary type and fetal gastrointestinal type [10].
AFP producing gastric tumour with isolated hepatoid morphology poses a diagnostic challenge when the primary presentation is liver metastases, as there is marked histologic and immunohistochemistry overlap. In such cases, Ushiku T et al., found that SALL4, an oncofetal protein to be positive in hepatoid gastric adenocarcinoma and negative in hepatocellular carcinoma [11]. Glypican 3 (GPC3), another oncofetal protein has also been found to be positive in all AFP producing gastric carcinomas with similar immune pattern as that of AFP [12]. GPC3 is not only a surrogate marker, but is also expected to be a target for forthcoming immunotherapy [12].
The exact cell of origin of this tumour is unclear. There are various proposed theories such as sequestered germ cells, retrodifferentiation from somatic cells or heterogenous differentiation of the same clone [13]. Similarly the factors contributing to the aggressive behaviour of these tumours is also unclear. A study conducted by Fujii H et al., found that these tumours arise from an aggressive clone with extensive loss of heterozygosity and high allelic loss [14].
In contrast to the aggressive behaviour of these tumours, there are a few case reports with good prognosis and prolonged disease free survival [15,16]. Similarly, our patient is also doing well without any evidence of metastases. This can probably be attributed to the early diagnosis and curative resection.
The present case is unique in the following aspects: (a) combination of varied histomorphologic patterns (hepatoid, yolk sac and papillary adenocarcinoma); (b) absent lymphovascular invasion with absent liver and lymph node metastases; (c) associated fundic gland polyps with varying degrees of dysplasia, well differentiated neuroendocrine tumours of stomach and duodenum and rectal ganglioneuroma; (d) prolonged disease free survival.
Conclusion
AFP producing gastric cancers although rare has now been well delineated. These tumours exhibit complex histologic picture. They are known to behave aggressively, hence a proper identification with precise subtyping and staging is necessary for appropriate treatment and in predicting the clinical behaviour.
[1]. Gitlin D, Perricelli A, Gitlin GM, Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptusCancer Res 1972 32(5):979-82. [Google Scholar]
[2]. O’Conor GT, Tatarinov YS, Abelev Gl, Uriel J, A collaborative study for the evaluation of a serologic test for Primary liver cancerCancer 1970 25:1091-98. [Google Scholar]
[3]. Motoyama T, Watanabe H, Yamamoto T, Sekiguchi M, Production of alpha-fetoprotein by human germ cell tumours in vivo and in vitroActa Pathol J Pn 1987 37:1263-77. [Google Scholar]
[4]. Yamagata T, Yamagata Y, Nakanishi M, Shirahama H, Tashiro Y, Iwasaki A, A case of primary lung cancer producing alpha-fetoproteinCan Respir J 2004 11:504-06. [Google Scholar]
[5]. Saito S, Hatano T, Hayakawa M, Koyama Y, Ohsawa A, Iwamasa T, Studies on alphafetoprotein produced by renal cell carcinomaCancer 1989 63:544-49. [Google Scholar]
[6]. Joo YH, Jung HY, Kang GH, Youn HJ, Kim SY, Yang SK, Clinico-pathological characteristics of the alpha-fetoprotein producing gastric carcinomaKorean J Gastroenterol 2000 36:54-60. [Google Scholar]
[7]. Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Clinicopathologic features and prognostic factors in alpha-fetoprotein- producing gastric cancers: analysis of 104 casesJ Surg Oncol 2010 102:249-55. [Google Scholar]
[8]. Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M, An AFP-producing gastric carcinoma with features of hepatic differentiation. A case reportCancer 1985 56:840-48. [Google Scholar]
[9]. Nagai E, Ueyama T, Yao T, Tsuneyoshi M, Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysisCancer 1993 72:1827-35. [Google Scholar]
[10]. Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K, Alpha-fetoprotein producing gastric carcinomas: a comparative study of three different subtypesActa Pathol Jpn 1993 43:654-61. [Google Scholar]
[11]. Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinomaAm J Surg Pathol 2010 34(4):533-40. [Google Scholar]
[12]. Ushiku T, Uozaki H, Shinozaki A, Ota S, Matsuzaka K, Nomura S, Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomasCancer Sci 2009 100(4):626-32. [Google Scholar]
[13]. Puglisi F, Damante G, Pizzolitto S, Mariuzzi L, Guerra S, Pellizzari L, Combined yolk sac tumour and adenocarcinoma in a gastric stump: molecular evidence of clonalityCancer 1999 85(9):1910-16. [Google Scholar]
[14]. Fujii H, Ichikawa K, Takagaki T, Nakanishi Y, Ikegami M, Hirose S, Genetic evolution of alpha fetoprotein producing gastric cancerJ Clin Pathol 2003 56(12):942-49. [Google Scholar]
[15]. Kim YS, Kim SH, Seong JK, Lee BS, Jeong HY, Song KS, Gastric yolk sac tumour: a case report and review of the literatureKorean J Intern Med 2009 24(2):143-46. [Google Scholar]
[16]. Geramizadeh B, Anbardar MH, Mashayekhi M, Alpha-fetoprotein producing gastric cancer with yolk sac component: two rare case reportsMiddle East Journal of Cancer 2016 7(2):109-11. [Google Scholar]