Heterotopic Prostate at Autopsy- An Unusual Mass at the Dome of the Urinary Bladder
Shital Munde1, Gwendolyn Fernandes2, Priyanka Phadnis3
1 Fellow, Department of Pathology, King Edward Memorial Hospital and Seth G.S. Medical College, Mumbai, Maharashtra, India.
2 Associate Professor, Department of Pathology, King Edward Memorial Hospital and Seth G.S. Medical College, Mumbai, Maharashtra, India.
3 Fellow, Department of Pathology, King Edward Memorial Hospital and Seth G.S. Medical College, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gwendolyn Fernandes, C-802, Swayam, Poonam Gardens, Mira Road, Mumbai-401107, Maharashtra, India.
E-mail: drgwenfern@yahoo.co.in
Heterotopic prostate in the dome of the urinary bladder is extremely rare and difficult to diagnose. It is often mistaken for neoplastic masses and histopathological examination is warranted for diagnosis. We report an autopsy case of an ectopic prostate at the dome of the bladder which mimicked a neoplasm on gross pathology.
Bladder mass, Ectopic prostate, Obstructive uropathy, Prostatic hyperplasia
Case Report
A 68-year-old male presented with fever and epigastric pain since three days. His previous medical records showed that he was diagnosed as chronic obstructive uropathy and chronic kidney disease resulting from an enlarged prostate. On enquiry, there was an occasional history of haematuria and there was a past history of pulmonary tuberculosis and chronic obstructive pulmonary disease with anti-tuberculous drug taken. On clinical examination, he was febrile and had epigastric tenderness. He also had a Foley catheter in situ. Laboratory investigations revealed haemoglobin 8.2 mg/dL and WBC count of 18,800/mcL with 88% neutrophils with toxic granules and band forms. Platelet count was normal and there was hypochromic microcytic anaemia. Serum creatinine was 2 mg/dL, Blood Urea Nitrogen (BUN) 42 mg/dL, Serum Glutamic Pyruvic Transaminase (SGPT) 36 U/L and Serum Glutamic Oxaloacetic Transaminase (SGOT) 37 U/L. Urine sediment examination showed abundant pus cells. Ultrasonography of abdomen showed a hypoechoic partially liquefied lesion within left lobe of liver, measuring 7 x 6 x 4 cm and a diagnosis of ruptured liver abscess was made. Ultrasonography of pelvis showed a urinary bladder with trabeculated walls and diverticulae suggestive of obstructive uropathy with bladder outflow obstruction. The prostate gland was enlarged in size and weighed 42 grams. Both the kidneys showed marked hydronephrosis and hydroureter. Ultrasound-guided catheter drainage of liver abscess was performed and pig tail catheter was inserted within the abscess cavity. Microbiological examination of drained pus was negative for microorganism and no growth was seen on the culture. He was treated with intravenous metronidazole, piperacillin-tazobactum, levofloxacin, tablet tamsulosin and deriphyllin. The patient had a six day hospital stay following which he succumbed to septicaemia and peritonitis.
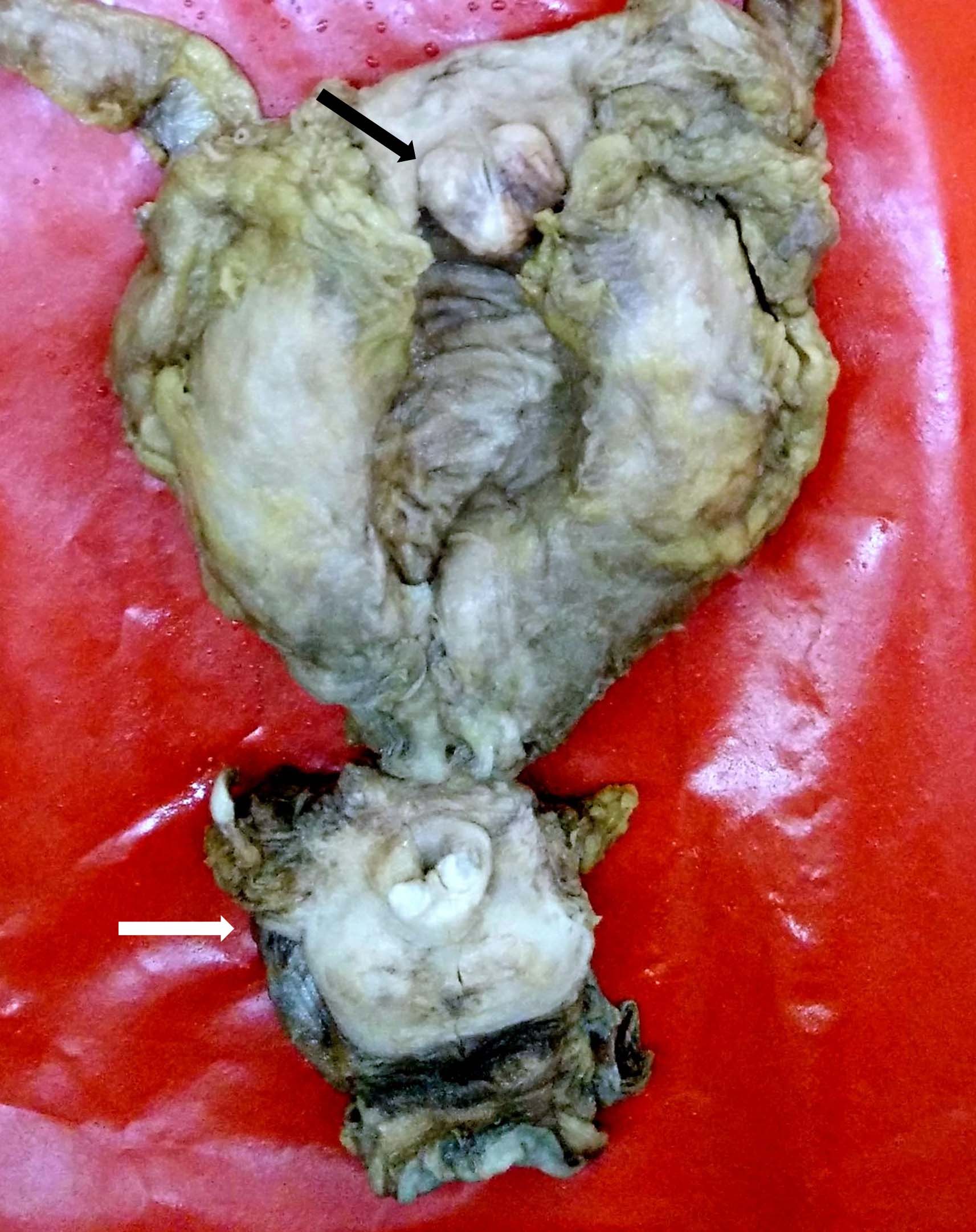
A complete autopsy was performed. The liver showed a large ruptured abscess measuring 10 x 5 cm. A 50 ml pus was present in the epigastric region and pus flecks were seen on the under surface of the diaphragm. The urinary bladder had trabeculations, mucosal erosions and showed a 3 x 2 x 2 cm firm, broad based, polypoidal mass at the dome projecting into the lumen [Table/Fig-1]. The cut surface of the mass showed a grey-white, whorled appearance. The gross impression was a neoplastic mass, probably a leiomyoma. The kidneys showed multiple scars on the external surface and hydronephrosis and hydroureter was confirmed.
Urinary bladder showing a 3x2x2 cm firm, broad based, polypoidal mass at the dome projecting in to the lumen (black arrow). Enlarged normal prostate gland also included the photograph (white arrow).
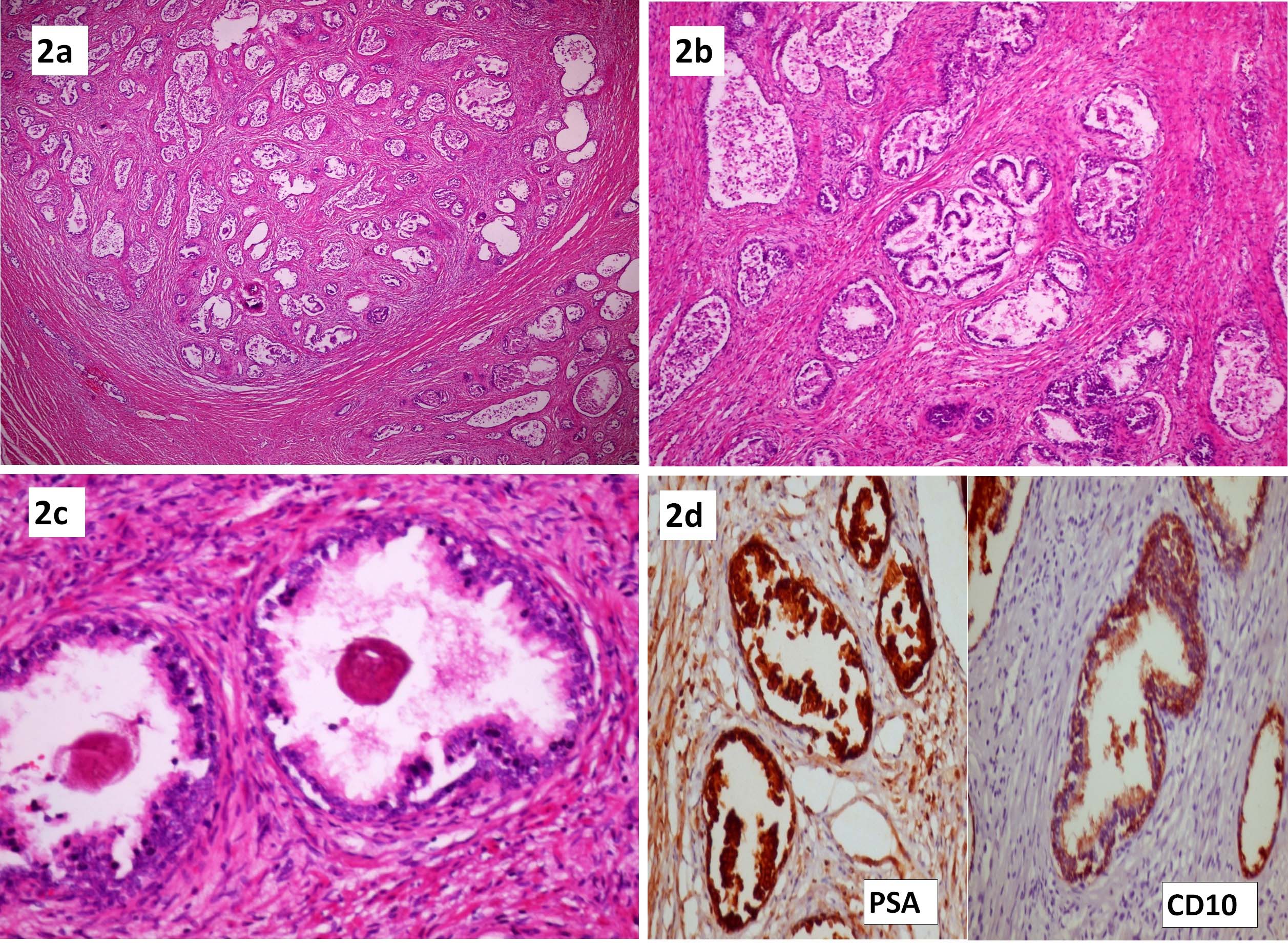
Histopathological examination of the mass in the urinary bladder showed well-formed prostatic tissue with complex tubular-alveolar glands embedded within the lamina propria and muscularis propria [Table/Fig-2a]. The glands were benign and showed two layers, a basal cell layer as well as a columnar cell layer with foamy cytoplasm and bland nuclear features. Many glands were cystically dilated with papillary infoldings [Table/Fig-2b]. Corpora amylacea was seen in many of the glands [Table/Fig-2c]. The overlying urothelium was thinned out and focally ulcerated. Immunohistochemistry was done to confirm the prostatic origin of the tissue and Prostate Specific Antigen (PSA) showed strong cytoplasmic positivity in the columnar cells while CD10 was positive in both columnar and basal cell layer [Table/Fig-2d]. A diagnosis of heterotopic prostate in the dome of the urinary bladder was made. This heterotopic prostate was histologically and immunohistochemically similar to the normal prostatic tissue. The normally located prostate gland showed features of nodular hyperplasia with cystically dilated glands and frequent papillary infoldings. The kidneys showed marked chronic pyelonephritis. Histopathological examination of the liver showed a ruptured tuberculous abscess composed of caseous necrotic material and dense neutrophilic infiltrate. The abscess was rimmed by florid epithelioid cell granulomas.
a) Well-formed prostatic tissue with complex tubular-alveolar glands embedded within the muscularis propria (H&E 4X); b) Cystically dilated benign glands with papillary infoldings (H&E 10X); c) Prostatic glands showing corpora amylacea within the lumen (H&E 40X). 2d: Immunohistochemistry- Prostatic specific antigen (PSA) and CD10 showing strong positivity (10X).
Discussion
Heterotopic or ectopic prostate is a rare and an underreported entity in urology practice. The first case of ectopic prostatic tissue was reported by Nicholson in 1922 and presented as a mass adjacent to the lower part of a patent urachal remnant [1]. The common site for heterotopic prostate is lower male genitourinary tract and very rarely it has also been reported in the female genitourinary tract. Two scattered case reports of heterotopic prostate in the anal canal, retroperitoneum and spleen are available in English literature [2]. The overall incidence of ectopic prostate reported till 2010 was genitourinary tract (31 cases), extragenitourinary sites (15 cases) and in females (16 cases) [3]. In the genitourinary tract, the most common sites for ectopic prostate are the posterior urethra (100 cases), near the verumontanum (12), bulbous urethra (7), bladder (13), posterior to bladder (3) and penile urethra (3) and this incidence has been reported in year 2004 [4]. In another study conducted in 2010 by Halat S et al., 20 cases of ectopic prostate in urinary tract were reported, the sites involved were bladder (17) and urethra (3) [2]. In the urinary bladder the commonest site was trigone and they also reported a single case of ectopic prostate located at the dome of urinary bladder [2]. Regarding the incidence of ectopic prostate in the urinary bladder, there are less than 50 cases reported in literature till date [5]. As far as the location of ectopic prostate at the dome of urinary bladder is considered, to the best of our knowledge five cases have been reported till date [6]. In our case, the heterotopic prostate was located at the dome of the urinary bladder and was projecting into the lumen as a polypoidal mass.
No definite aetiological factors has been attributed to this entity and different theories for heterotopic prostate have been suggested which includes persistence of embryonic remnants, migration and misplacement of normal tissue and metaplastic change caused by chronic inflammation [7,8]. Persistence of embryonic remnants is the most favoured theory and the migration theory is favoured wherever there was no histological evidence of inflammation or metaplastic processes in the lesion [6].
The clinical features reported include gross or microscopic haematuria, dysuria and persistent urinary tract infections [3,6]. In our patient, the heterotopic prostate was probably the cause of haematuria, however the normally located prostate showed prostatomegaly, features of nodular prostatic hyperplasia on histology and clinical presentation of obstructive uropathy along with complications like trabeculations and diverticulae of the urinary bladder. The heterotopic prostate could not be diagnosed during life as the patient was catheterised for chronic obstructive uropathy and the bladder could not be filled to capacity for proper ultrasound examination.
Grossly, heterotopic prostate present as firm, smooth, sessile bladder masses, as was seen as in our case. Corpora amylacea is helpful in identifying prostatic glands. Heterotopic prostate may also show features of hyperplasia and chronic prostatitis similar to normal prostate. Two cases of prostatic adenocarcinoma arising in heterotopic prostatic tissue has been reported [3].
Immunohistochemistry with PSA and Prostate Specific Acid Phosphatase (PSAP) in conjunction with other markers for prostate tissue are used for confirmation of ectopic prostate [2]. CD10 is considered a marker for tissues of mesonephric origin and is reported to be positive in the epithelial and basal cells of normal and hyperplastic prostatic acini [9]. In our case, the presence of corpora amylacea within the glands and strong immunohistochemistry staining for PSA and CD10 is confirmatory and does not leave any scope for differential diagnosis. Heterotopic prostate is benign and surgical excision is curative [2].
Conclusion
Heterotopic prostate at the dome of the urinary bladder, though very rare, is difficult to diagnose and is often mistaken on cystoscopy for bladder tumors. Histopathological examination clinches the diagnosis and helps misdiagnosis from sinister lesions. Also whenever prostatic type epithelium or glands are seen away from their anatomic location the possibility of heterotopic prostate tissue should be thought of.
[1]. Nicholson GW, The Heteromorphoses in the Human BodyGuy’: Hosp. Rep 1922 Jan Lxxn:75-127. [Google Scholar]
[2]. Halat S, Eble JN, Grignon DJ, Lacy S, Montironi R, MacLennan GT, Ectopic prostatic tissue: histogenesis and histopathological characteristicsHistopathology 2011 58:750-58. [Google Scholar]
[3]. Gardner JM, Khurana H, Leach FS, Ayala AG, Zhai J, Ro JY, Adenocarcinoma in ectopic prostatic tissue at dome of bladder: a case report of a patient with urothelial carcinoma of the bladder and adenocarcinoma of the prostateArch Pathol Lab Med 2010 134:1271-75. [Google Scholar]
[4]. Trivedi V D, Salve S A, Dangle P, Sawant A, Gadgil Nelivegi G, Ectopic prostatic tissue presenting as a bladder massIndian J Urol 2004 20:167-68. [Google Scholar]
[5]. Chung SD, Sun HD, Hsiao CH, Ectopic Prostate in the Urinary BladderIncont Pelvic Floor Dysfunct 2008 2(4):165 [Google Scholar]
[6]. Kim JH, Jeen YM, Song YS, Ectopic prostate tissue at the bladder dome presenting as a bladder tumorWorld J Mens Health 2013 31(2):176-78. [Google Scholar]
[7]. Wallace C, Creager AJ, Cappellari JO, Bergman S, Ectopic prostatic tissue in the uterine cerrixAm J Surg Pathol 2001 25:1215-16. [Google Scholar]
[8]. Roy C, Guth S, Gasser B, Le Bras Y, Tuchmann C, Saussine C, Benign hyperplasia in ectopic prostatic tissue: a rare cause of pelvic massEur Radiol 1997 7:35-37. [Google Scholar]
[9]. Tawfic S, Niehans GA, Manivel JC, The pattern of CD10 expression in selected pathologic entities of the prostate glandHum Pathol 2003 34:450-56. [Google Scholar]