AS along with other conditions having predominant axial involvement and key symptoms of both inflammatory back pain and stiffness (such as psoriatic arthritis and non-radiographic axial spondyloarthritis) are usually grouped together as Axial Spondylo-Arthritis (ASpA) [1].
AS is a chronic inflammatory disease primarily affecting the sacroiliac joints and axial skeleton, and involving significant reduction in spinal mobility. The monitoring of disease activity in AS is critical for assessing the response to treatment. Many tools and validated scores are available for this purpose, including the Bath AS Disease Activity Index (BASDAI) [2], the Bath AS functional index (BASFI) [3], the Bath AS Metrology Index (BASMI) [4], and the AS Disease Activity Score (ASDAS) [5].
The ASDAS was first published by the Assessment in Ankylosing Spondylitis (ASAS) group in 2008 [5]. ASDAS includes both patient-reported components (back pain, duration of morning stiffness, peripheral joint pain and/or swelling, and general well-being) and an objective serologic marker of inflammation (ESR or CRP) [6].
We decided to develop a simplified version of the ASDAS which may be useful and a cost-effective tool for monitoring disease activity in Asian Indian patients with AS.
Materials and Methods
This study included Asian Indian patients diagnosed with AS according to the modified New York [11] and/or Assessment in Ankylosing Spondylitis 2011 criteria [12]. Consenting consecutive ambulatory patients of either gender aged >18 years attending the Rheumatology outpatient department of Sir Ganga Ram Institute, New Delhi, India, between January 2012 and December 2014 were recruited in the study. The study protocol was approved by the Institutional Ethical Committee, which worked according to the ethical standards described in the Helsinki Declaration of 1975 (revised in 2000).
Baseline data collected included sociodemographic data, disease duration, presence of comorbidities, HLA-B27 status, ESR by Westergren’s method, and CRP by nephelometry. BASDAI, BASFI, and Ankylosing Spondylitis Quality of Life (ASQoL) [6] were collected, and ASDAS-ESR and ASDAS-CRP were calculated according to the formula provided by the ASDAS [13].
The Simplified ASDAS (SASDAS) was calculated as the simple sum of patient global assessment {using Visual Analogue Scale (VAS), 0-10}, back pain (BASDAI question no. 2, 0-10), peripheral pain and swelling (BASDAI question no. 3, 0-10), morning stiffness (BASDAI question no. 6, 0-10), and either ESR in millimetres per hour (for SASDAS-ESR) or, CRP in mg/L (for SASDAS-CRP); this sum was divided by 10 to obtain the final score [Table/Fig-1].
Calculation of SASDAS-ESR and SASDAS-CRP.
| SASDAS-ESR | SASDAS-CRP |
|---|
| Simple sum of:1. Patient global assessment (using VAS, 0-10)2. Back pain (BASDAI question no. 2, 0-10)3. Peripheral pain and swelling (BASDAI question no. 3, 0-10)4. Morning stiffness (BASDAI question no. 6, 0-10)5. ESR in mm/hrDivide the sum by 10 | Simple sum of:1. Patient global assessment (using VAS, 0-10)2. Back pain (BASDAI question no. 2, 0-10)3. Peripheral pain and swelling (BASDAI question no. 3, 0-10)4. Morning stiffness (BASDAI question no. 6, 0-10)5. CRP in mg/LDivide the sum by 10 |
VAS= Visual Analogue Scale.
Statistical Analysis
All of the data was entered electronically. Statistical analysis was performed using SPSS version 22.0. Spearman test was used to assess correlation between SASDAS and ASDAS. Stepwise linear regression was performed to assess the correlation of SASDAS-ESR and SASDAS-CRP with other variables. The cut-off values for the SASDAS-ESR and SASDAS-CRP for different stages of the disease were analysed and determined using Receiver Operating Characteristic (ROC) curves. A p-value<0.05 was considered significant for all tests applied.
Results
A total of 254 patients (224 males and 30 females) were included in the study. Mean age of the affected patients at the time of diagnosis was 32.98±11.82 years (range 16 to 74 years, median 30 years) while the mean age of onset was 27.29±10.13 years. So, the average delay in diagnosis came out to be 5.704±5.21 years (two months to 40 years). Overall, one or more peripheral joints were involved in 129/254 (50.7%) of patients. Most common extra-articular presentation was acute anterior uveitis (34/254, 13.38%). Other comorbidities reported include chronic kidney disease, type 2 Diabetes, hypertension and anaemia (1 patient each). HLA-B27 was positive in 230/254 patients (90.55%).
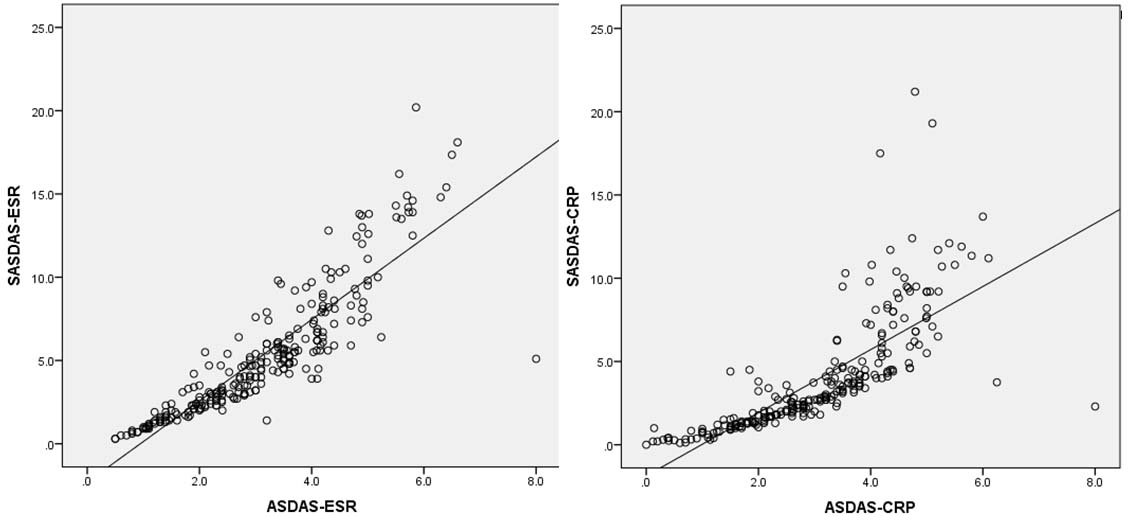
The correlation between SASDAS and ASDAS is showed in [Table/Fig-2]. SASDAS-ESR and SASDAS-CRP showed good correlation with the ASDAS-ESR and ASDAS-CRP respectively (Spearman’s rho 0.93 and 0.92 respectively; p-value<0.001).
Correlation of SASDAS-ESR with ASDAS-ESR, and SASDAS-CRP with ASDAS-CRP.
The results of the stepwise linear regression of SASDAS-ESR and SASDAS-CRP with other variables are summarised in [Table/Fig-3]. SASDAS-ESR showed good correlation with CRP (r=0.50) whereas with it was fairly correlated with backpain (r=0.19), morning stiffness (r=0.21) and peripheral pain (r=0.21); SASDAS-CRP showed good correlation with BASFI (r=0.32) and ESR (r=0.55) (all p-value<0.05).
Correlation of SASDAS-ESR and SASDAS-CRP with measures of disease assessment using stepwise linear regression.
| Variables | SASDAS-ESR | SASDAS-CRP |
|---|
| r | p | r | P |
| Age | 0.112 | 0.004 | -- | 0.446 |
| Gender | -- | 0.364 | -- | 0.139 |
| HLA-B27 status | -- | 0.934 | -- | 0.487 |
| Disease duration | -- | 0.179 | -- | 0.897 |
| Back pain (VAS) | 0.197 | <0.0001 | -- | 0.214 |
| Morning stiffness (VAS) | 0.207 | <0.0001 | -- | 0.943 |
| Peripheral pain (VAS) | 0.212 | <0.0001 | -- | 0.126 |
| BASFI | -- | 0.125 | 0.318 | <0.0001 |
| ASQoL | -- | 0.072 | -- | 0.587 |
| Fatigue score (VAS) | -- | 0.286 | -- | 0.706 |
| ESR | -- | -- | 0.551 | <0.0001 |
| CRP | 0.501 | <0.0001 | -- | -- |
ESR=Erythrocyte Sedimentation Rate; CRP=C-Reactive Protein; VAS=Visual Analogue Scale; ASQoL=Ankylosing Spondylitis Quality of Life; BASFI=Bath Ankylosing Spondylitis Functional Index.
Patients were divided into AS disease categories (inactive, moderate, high, and very high disease activity) based on their ASDAS-ESR and ASDAS-CRP scores, using established ASDAS cut-off values as suggested by ASAS [13]. Using ROC curves, the corresponding cut-off points were determined at 1.83, 2.45 and 4.45 for SASDAS-ESR, and 0.79, 1.50, and 3.26 for SASDAS-CRP. The cut-off points showed a high discriminative capacity with high sensitivity and specificity, as summarised in [Table/Fig-4].
Cut-off points and their sensitivity-specificity values for SASDAS with respect to disease activity.
| Disease activity | SASDAS-ESR cut-off point | Sensitivity | Specificity | SASDAS-CRP cut-off point | Sensitivity | Specificity |
|---|
| Inactive to moderate | 1.83 | 92% | 96% | 0.79 | 99% | 84% |
| Moderate to high | 2.45 | 95% | 89% | 1.50 | 98% | 90% |
| High to very high | 4.45 | 97% | 80% | 3.26 | 96% | 90% |
When ASDAS and SASDAS were cross-tabulated, it was found out that SASDAS-ESR agrees better with ASDAS-ESR in the extremes of the condition [Table/Fig-5], whereas SASDAS-CRP agrees with ASDAS-CRP throughout the disease activity [Table/Fig-6].
Cross tabulation of disease activity categories of SASDAS-ESR and ASDAS-ESR.
| | ASDAS-ESR disease activity categories | Total |
|---|
| Inactive(<1.3) | Moderate(1.4-2.1) | High(2.2-3.4) | Very high(>3.5) |
|---|
| SASDAS-ESR disease activity categories | Inactive(<1.83) | 21(95.5%) | 17(44.7%) | 1(1.1%) | 0 (0%) | 39(15.4%) |
| Moderate(1.84-2.45) | 1(4.5%) | 14(36.8%) | 8(9.0%) | 0 (0%) | 23(9.1%) |
| High(2.46-4.45) | 0 (0%) | 7(18.4%) | 49(55.1) | 3(2.9%) | 59(23.2%) |
| Very high(>4.46) | 0(0%) | 0 (0%) | 31(34.8%) | 102(97.1%) | 133(52.4%) |
| Total | 22(100%) | 38(100%) | 89(100%) | 105(100%) | 254(100%) |
Note: Values are N (%).
Cross tabulation of disease activity categories of SASDAS-CRP and ASDAS-CRP.
| | ASDAS-CRP disease activity categories | Total |
|---|
| Inactive(<1.3) | Moderate(1.4-2.1) | High(2.2-3.4) | Very high(>3.5) |
|---|
| SASDAS-CRP disease activity categories | Inactive(<0.79) | 20(83.3%) | 1(2.4%) | 0(0%) | 0(0%) | 21(8.3%) |
| Moderate(0.80- 1.5) | 4(16.7%) | 32(78%) | 4(4.6%) | 0(0%) | 40(15.7%) |
| High(1.51- 3.26) | 0(0%) | 5(12.2%) | 70(80.5%) | 4(3.9%) | 79(31.1%) |
| Very high(>3.26) | 0(0%) | 3(7.3%) | 13(14.9%) | 98(96.1%) | 114(44.9%) |
| Total | 24(100%) | 41(100%) | 87(100%) | 102(100%) | 254(100%) |
Note: Values are N (%).
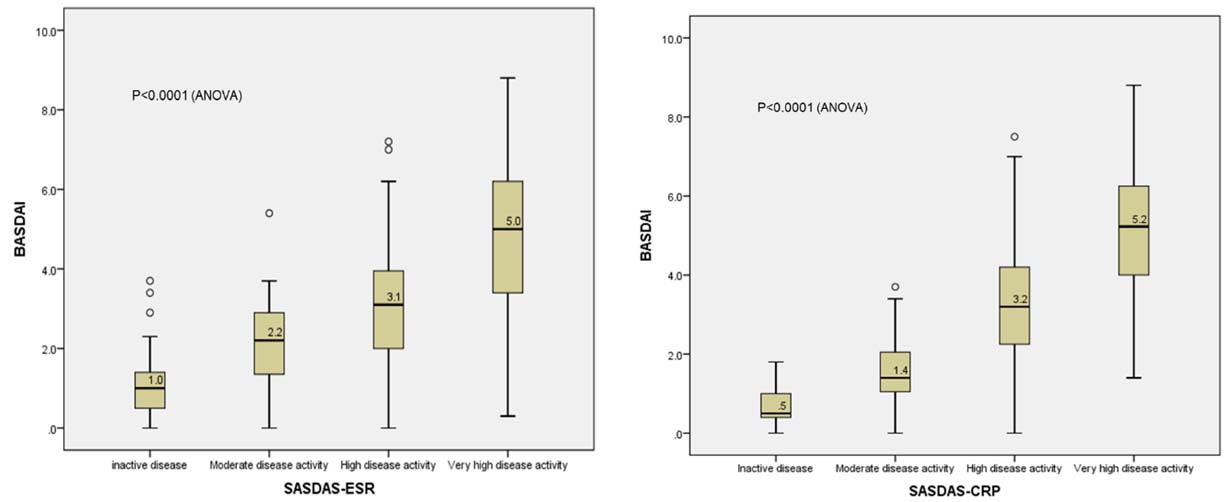
When the distribution of BASDAI was analysed in relation to different SASDAS cut-off points, an upward distribution with a significant difference among the disease stages was observed with both SASDAS-ESR and SASDAS-CRP (p-value<0.001 in both cases; ANOVA) [Table/Fig-7].
Distribution of BASDAI with respect to SASDAS-ESR and SASDAS-CRP cut-off points.
Discussion
AS is a chronic inflammatory disorder affecting the spine and entheses, occasionally accompanied with skeletal and extra-skeletal tissue affectations. It is most commonly seen in young men, and leads to a significant loss in productivity [14].
The last decade has seen significant development in the diagnosis and management of AS, and the major contributors for these developments include development of newer imaging techniques and several new therapies for AS. According to the 2015 ACR/SAA/SPARTAN (American College of Rheumatology/Spondylitis Association of America/ Spondylo-arthritis Research and Treatment Network) recommendations, the first line of therapy for AS involves NSAIDs with non-pharmacological treatment modalities. In case of non-response to, intolerance to, or disease progression despite NSAIDs, biological response modulators targeting Tumour Necrosis Factor-α (TNF-α) may be initiated as second line of therapy [15].
The disease activity of AS is a direct pointer towards the effectiveness of treatment, suggests the level of inflammation, directs treatment decisions, and predicts the prognosis of the condition [14]. A properly validated tool to measure disease activity in AS is also required for validation of clinical trials evaluating drugs in AS management. Such a validated tool can effectively delineate the trial population of interest and can objectively measure the outcome of clinical trials.
One of the most widely accepted and extensively validated tool for measuring disease activity in AS worldwide is the BASDAI [16]. However, the BASDAI is a questionnaire-based tool, and collects patient-specific and subjective information. Thus, it cannot represent the whole spectrum of disease activity in AS [10]. Other tools such as BASFI and ASQoL also have the same limitation. On the other hand, the BASMI is a score which exclusively involves objective assessment of the patient’s progress in AS, without accounting for the patient-centred and subjective aspects of AS. Compared to these, the ASDAS has subjective as well as objective components (in the form of ESR or CRP) [6].
The main problem with ASDAS is that it is cumbersome to use. The formula for calculating ASDAS, as endorsed by ASAS, is complicated: ASDAS-CRP = 0.12 x Back Pain + 0.06 x Duration of Morning Stiffness + 0.11 x Patient Global + 0.07 x Peripheral Pain/Swelling + 0.58 x Ln (CRP+1); ASDAS-ESR = 0.08 x Back Pain + 0.07 x Duration of Morning Stiffness + 0.11 x Patient Global + 0.09 x Peripheral Pain/Swelling + 0.29 x square root of ESR [13].
This prompted investigators to evaluate scores with both subjective and objective elements that are easier to calculate. The SASDAS, as published by Sommerfleck FA et al., is one such attempt at this [10]. This SASDAS was calculated by simple linear addition of five components: patient global assessment (VAS 0–10 cm), back pain (BASDAI question no. 2, 0–10 cm), peripheral pain and swelling (BASDAI question no. 3, 0–10 cm), duration of morning stiffness (BASDAI question no. 6, 0–10 cm), and ESR in millimetres per hour, divided by 10. This new scale was found out to have ‘comparable internal and external responsiveness with respect to the ASDAS ESR/CRP response criteria’ in these patients [9]. However, a recent study suggests that this SASDAS showed good agreement with ASDAS-ESR and only a moderate degree of agreement with ASDAS-CRP [17]. This is an issue of concern because, according to the ASAS, the ASDAS-CRP is the ‘preferred’ formula and the ASDAS-ESR is the ‘alternative’ [13]. The main limitation of the above mentioned studies is using only ESR in calculation of SASDAS whereas ASDAS-CRP is the preferred method. There was no comparison between SASDAS-CRP with other disease activity scores in previous studies.
In our study, we have come up with a slight modification over this SASDAS in that, we have taken ESR and CRP separately and compared both. We found out that our SASDAS-CRP correlated with the standard ASDAS-CRP across all categories of the disease, whereas our SASDAS-ESR correlated with the standard ASDAS-ESR only at the extremes of the disease. Both SASDAS-ESR and SASDAS-CRP have shown excellent correlation with ASDAS-ESR and ASDAS-CRP respectively, and also with BASDAI which is a reliable measure of disease activity. With these results, it appears that though the cut-off points for SASDAS-ESR have shown a high degree of sensitivity and specificity, they seem to agree with the established ASDAS-ESR disease activity categories only at the extremes of the disease. On the other hand, the SASDAS-CRP in addition to showing a high degree of sensitivity and specificity, also agrees with the established ASDAS-CRP disease activity categories.
These cut-off values were different from parameters established by Salaffi F et al. and Sommerfleck FA et al., [9,10]. The major difference seems to be primarily due to differences in estimation and normal cut-off values of ESR and CRP. In most labs in India, CRP is calculated as mg/L, and 5-6 mg/L is the normal cut-off value. In contrast, CRP is estimated in mg/dl in the European countries, and the normal value is <1 mg/dl. Moreover, when compared to ESR, CRP has better relationship with inflammation and disease activity. Finally, ESR is prone to be affected by many physiological conditions. This is the reason why ASAS group has established ASDAS-CRP as preferred score and ASDAS-ESR as the alternative.
Limitation
Though our study has included 254 Asian Indian AS patients (predominantly from Northern India), these results should be confirmed by further studies involving larger samples and from diverse populations across India. Also, there is a need for a prospective study to establish internal and external responsiveness of SASDAS-ESR and SASDAS-CRP in monitoring AS disease activity in Indian patients.
Conclusion
The SASDAS-CRP (better than SASDAS-ESR) can be a useful and easy-to-calculate tool for routine assessment of disease activity in daily clinical practice. However, SASDAS- CRP is less cost effective when compared to SASDAS-ESR, and this factor may be an important issue in cost constrained countries with limited resources especially where quantitative estimation of CRP by nephelometry is not available. In this setting, SASDAS-ESR is still an easy, feasible and cost-effective tool for measuring disease activity in AS and is comparable to ASDAS-ESR.
VAS= Visual Analogue Scale.
ESR=Erythrocyte Sedimentation Rate; CRP=C-Reactive Protein; VAS=Visual Analogue Scale; ASQoL=Ankylosing Spondylitis Quality of Life; BASFI=Bath Ankylosing Spondylitis Functional Index.
Note: Values are N (%).
Note: Values are N (%).