Bone Marrow-derived Mesenchymal Stromal Cells (BM-MSCs) are adult stem cells. Mesenchymal Stem Cells (MSCs) are a heterogeneous subset of stromal stem cell population that can be isolated from various sources of adult tissues such as bone marrow, adipose tissue, placenta, umbilical cord and cord blood, dental pulp, and amnion. Bone Marrow (BM) remains the primary source of MSCs for most of the preclinical and clinical studies [1]. The secretion of bioactive molecules by MSCs, such as growth factors, cytokines, and chemokines, contributes to their biologically substantial activity. MSCs are generally considered to exhibit four major properties: (a) capacity for self-renewal or self-maintenance; (b) multipotency; (c) functional, long-term tissue reconstitution; and (d) serial transplantability without immune rejection [2,3]. These properties of MSCs make them potentially ideal candidates for regenerative medicine, tissue engineering, and immunotherapy. Although MSCs transplantation is considered safe [4] and has been widely tested in clinical trials, many studies have also reported risks associated with the use of MSCs [5]. Studies of cell transplantation therapies in animal models are often hampered by the partial or complete rejection of grafts. Also, some studies reported that MSCs could undergo many spontaneous transformations, with tumourigenic potential [6]. For the above-mentioned reasons, preclinical safety testing of BMMSCs should be completed before their use in clinical trials. During our literature review, it was found that a standardized approach has not yet been established to evaluate the safety and toxicity of MSCs in vivo. Feeding, drinking, and body weight are interrelated and are considered as critical variables in many types of experiments involving the dosage of pharmacological agents [7]. Hence, these parameters were assessed in the present study in which, preclinical safety and toxicological studies were performed using two doses of BM-MSCs in Wistar rats.
Materials and Methods
Experimental Animals
This is an experimental study performed between June 2016 to October 2016. Eighteen female Wistar albino rats (4–5-month-old), weighing 140–160 gram were selected for this study. Animals were bred locally in the central animal house of Manipal University, Manipal, Karnataka, India. Animals were housed individually in polypropylene cages containing sterile paddy husk (procured locally) as bedding and maintained under standard conditions with temperature (22-24°C), 12 hours light/12 hours dark cycle, and relative air humidity 40%-60%. The animals were accustomed to the laboratory conditions for one week before the start of the experiment. Rats had continuous access to standard rat pellet diet and to tap water. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC/KMC/20/2014), and experiments were conducted according to the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA).
Isolation and Culture of BM-MSCs
Bone marrow aspirates were obtained from bone marrow samples of healthy adult males aged 20–35 years, after signed informed consent and under institutional ethical guidelines. BM-MSCs were isolated and expanded using a method previously reported by Pal R et al., [8]. The pooled MSCs from the working cell bank were further grown in serum-containing media and expanded. Freshly-thawed BM-MSCs suspended in Plasmalyte A was used for injecting intravenously into the rats.
Experimental Design
Animals were randomly divided into three groups comprising of six animals in each group. Animals were housed individually to measure the food and water intake of each rat per day. Group 1 – Normal control group, Group 2 – Low dose MSCs group (received 3.25 million human equivalent dose of BM-MSCs/kg body weight), Group 3 – High dose MSCs group (received 9.75 million human equivalent dose of BM-MSCs/kg body weight). The cell number used in this study was chosen by a dose optimization pilot study performed previously [9]. Both the doses of MSCs were formulated in 0.5 ml Plasmalyte A and injected intravenously into the tail vein of the rat. Cells were administered slowly and carefully to avoid embolism. The study period was thirty days.
Safety Assessment
During the study period, we observed all animals for changes in behaviour like grooming, sleeping pattern, general clinical signs like inactivity, lethargy and presence of any abnormal response, mortality, body weight, food consumption and water intake. Behaviour was continuously monitored for one hour using methods adapted from Fray PJ et al., [10]. Body weight of each animal in the three groups was recorded at the start of the experiment and at the end of the 6th, 12th, 18th, 24th and 30th day. Twenty-four-hour food and water intake of each rat was measured daily for 30 days. Food pellets were weighed and kept in each cage. Cages were inspected daily to check for loose pellets. Water was kept in plastic bottles with gradations. Average water and food intake/day at the end of 6th, 12th, 18th, 24th and 30th day was used for subsequent analysis.
Statistical Analysis
Using Statistical Package for the Social Sciences (SPSS version 16.0), data were expressed as mean±standard error of mean. Statistical differences between means of different groups were determined using repeated measures Analysis of Variance (ANOVA) followed by Tukey’s post hoc test. The p<0.05 was considered as statistically significant.
Results
Repeated measures ANOVA with a Greenhouse Geisser correction determined that the mean body weight, mean food intake, mean water intake for animals of all the three groups differed significantly between the time points (days), (p<0.05). Significant increase in animal body weights, food and water intake at all weeks compared to week zero was observed.
Effect of BM-MSCs on Body Weight
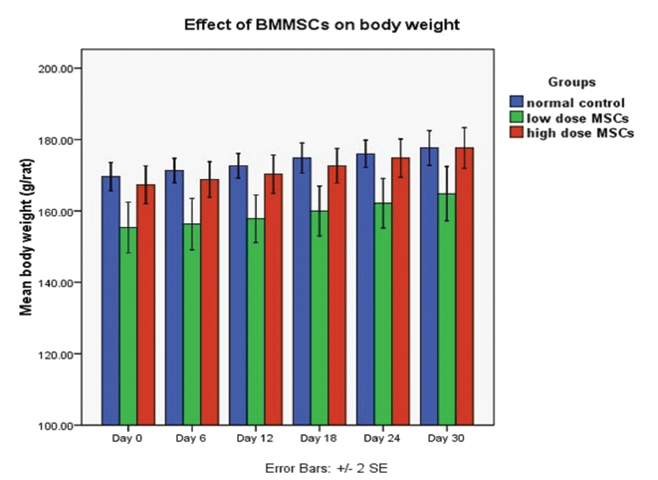
There was no significant difference in body weight of the animals at the beginning of the study. Over the study period, gradual increase in body weight of all the animals was observed. Absolute body weight of animals at the end of the 30th day was not statistically significant between normal control and MSCs groups. However, between the low dose and high dose MSCs group, absolute body weight at the end of the 30th day was statistically significant (p=0.01) [Table/Fig-1,2].
Table showing the mean body weight increase of rats after intravenous administration of mesenchymal stromal cells in the 30-day safety study.
| Groups (n=6) | Mean body weight (gm) on day 0 | Mean body weight (gm) on day 30 |
|---|
| Normal control | 169.6 ± 4.8 | 177.6 ± 5.9 |
|---|
| Low dose BM-MSCs | 155.3 ± 8.7 | 164.8 ± 9.3 |
| High dose BM-MSCs | 167.3 ±6.5 | 177.6 ± 7.0* |
Values are expressed as mean±SEM. Tukey’s post hoc test indicated statistically significant difference in body weights of high dose BMMSCs group when compared to low dose BMMSCs group on day 30, (* p<0.05). n: Number of rats in each group. BMMSCs: Bone Marrow-derived Mesenchymal Stromal Cells.
Graph showing the effect of low and high dose BMMSCs transplantation on body weight (gm) of rats.
Each bar represents mean±SEM from six rats. Repeated measures ANOVA with Greenhouse-Geisser corrections were used to analyze the data. Given the results of this correction, the difference in body weights of rats overtime (days) in each of the groups was statistically significant (p<0.05)
Effect of BM-MSCs on Behaviour
To examine the general behavioural pattern, behaviours were grouped into three categories: activity (rearing, burrowing, and locomotion); grooming; and resting. There were no significant changes observed in these behavioural patterns in the MSCs injected groups when compared to the normal control group which indicated that MSCs did not alter the general behaviour in rats.
Effect of BM-MSCs on Food and Water Intake
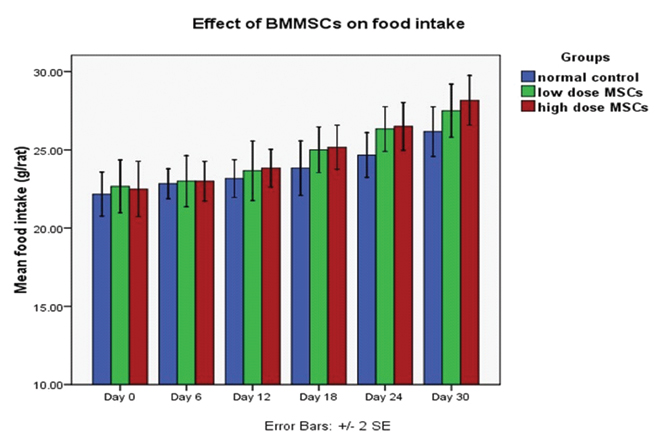
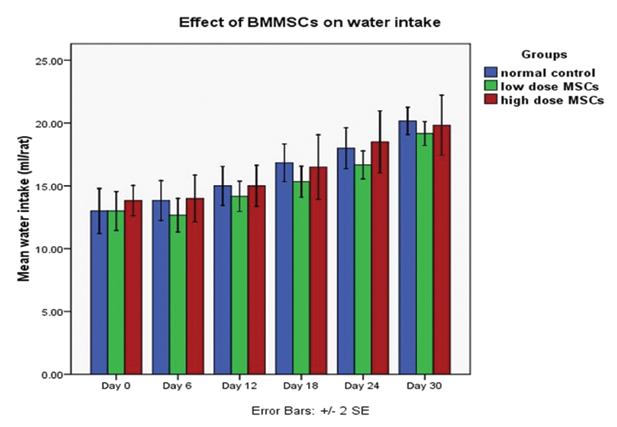
There were no significant differences in 24 hour food and water intake of animals between control and MSCs groups at the end of the 30th day study period (p>0.05). Average 24 hour food intake of the animals at the end of the study period was 26.2±1.9 gm for normal control, 27.5±2.1 gm for low dose MSCs group and 28.2±1.9 gm for high dose MSCs group [Table/Fig-3,4]. Average 24 hour water intake of the animals at the end of the study period was 20.1±1.3 ml for normal control, 19.1±1.2 ml for low dose MSCs group and 19.8±2.9 ml for high dose MSCs group [Table/Fig-3,5]. There was no statistically significant difference in food and water intake of animals among the MSCs injected groups and normal control group (p>0.05).
Table showing the mean food and water intake of rats after intravenous administration of mesenchymal stromal cells in the 30-day safety study.
| Groups (n=6) | Mean 24 hour food intake (gm) | Mean 24 hour water intake (ml) |
|---|
| Day 0 | Day 30 | Day 0 | Day 30 |
|---|
| Normal control | 22.1 ± 1.7 | 26.2 ± 1.9 | 13.0 ± 2.1 | 20.1 ± 1.3 |
| Low dose BM-MSCs | 22.6 ± 2.1 | 27.5 ± 2.1 | 13.0 ± 1.8 | 19.1 ± 1.2 |
| High dose BM-MSCs | 22.5 ± 2.1 | 28.2 ± 1.9 | 13.8 ± 1.4 | 19.8 ± 2.9 |
Values are expressed as mean±SEM deviation. Tukey’s post hoc test indicated no statistically significant difference in food and water intake between normal control group and BMMSCs group on day 30, (p>0.05). n: Number of rats in each group. BMMSCs: Bone Marrow-derived Mesenchymal Stromal Cells.
Graph showing the effect of low and high dose BMMSCs transplantation on food intake (gm) of rats.
Each bar represents mean±SEM from six rats. Repeated measures ANOVA with Greenhouse-Geisser corrections were used to analyze the data. Given the results of this correction, the difference in food intake of rats over time (days) in each of the groups was statistically significant (p<0.05).
Graph showing the effect of low and high dose BMMSCs transplantation on water intake (ml) of rats.
Each bar represents mean±SEM from six rats. Repeated measures ANOVA with Greenhouse-Geisser corrections were used to analyze the data. Given the results of this correction, the difference in water intake of rats over time (days) in each of the groups was statistically significant (p<0.05).
Effect of BM-MSCs on Mortality and Morbidity
All the 18 animals survived for the duration of the study. Further, there was no adverse clinical signs or symptoms or complications following the MSCs treatment which indicated that the administration of MSCs was safe.
Discussion
The efficacy and safety of BM-MSCs treatments must be established in appropriate animal models before clinical application in humans. During our literature review we noticed that there is paucity of data regarding the safety assessment of stem cells especially human bone marrow derived stromal cells in vivo. Hence, this study was carried out to assess the safety of two doses (low dose, high dose) of BM-MSCs in Wistar rats.
Although MSCs are considered to be safe, several studies have also reported risks associated with the use of mesenchymal stem cells. The tumourigenic potential of MSCs has been reported in; in vitro and in vivo experimental settings using stem cells obtained from different sources [11-13]. Hence, it has been postulated that, preclinical safety testing of MSCs should be completed before their use in clinical trials.
The results of the present study indicate, that intravenous administration of BM-MSCs is safe without any detectable side effects like changes in behaviour or mortality. Similar finding was also observed in a study done by Choi HJ et al., [14]. They found that transplantation of human adipose tissue-derived mesenchymal stem cells in rats is safe in terms of development of tumour and mortality. In another study, systemic administration of allogenic BM-MSCs was found safe with no change in the overall health or immune status of the rat. [15]. A study of stem cells in a rat model of middle cerebral artery occlusion reported that, the greatest therapeutic benefit of MSCs was obtained when a single high cell dose injection of MSCs was used, rather than multiple infusions of smaller cell doses over several time points [16]. Hence, in here, we gave a single infusion of MSCs to study its safety.
Another important observation of the study was that the animals injected with low and high dose of MSCs survived during the entire study. Although no significant difference was found in body weights, food, and water intake of the animals in the normal and MSCs injected group, gradual increase in these parameters was seen as the time progresses. Between the low dose and high dose MSCs group, absolute body weight at the end of the study was statistically significant. This difference was thought to be due to intermittent changes rather than injection dose. Average food intake of the animals was found to be 26-28 gm/rat/day and average water intake of the animals was 19-20 ml/rat/day, which is in accordance with a previous study [17]. This shows that the BM-MSCs did not alter the food and water intake, behaviour of the animals and did not have any negative effect on its body weight.
Limitation
There are several limitations of this study. We could not explore other effects of BM-MSCs like their tumourigenic potential, immune reactions, and histological changes due to lack of standardized protocols. Further investigations will be done for closer evaluation of the mechanism of MSCs action and other variables.
Conclusion
The results of the safety assessment performed in this study indicate that administration of BM-MSCs is safe when given in a single dose and by slow intravenous infusion. This study provides a background for standardization and application of regulatory requirements for emerging stem cell therapies.