Acute bacterial meningitis is the most common infection of CNS and remains a major cause of mortality and morbidity. The number of estimated cases of bacterial meningitis is 1.2 million each year worldwide. The case fatality rate is 16%-32% in India and other developing countries [1]. The causative agents vary among the age groups and geographical regions. But till recently, more than 80% of the cases were due to the triad - Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis, which were transmitted through respiratory secretions. Their prevalence decreased after introduction of vaccines (from 45% to a recent estimate of 7% in case of H. influenzae type b) [2]. However, in India S. pneumoniae is the predominant pathogen in children and adults [1]. N. meningitidis is predominant in Northern India and is very uncommon in the Southern states [3].
Clinical signs of meningitis are not always reliable, and laboratory support is necessary to establish an aetiological diagnosis. Conventional methods like Gram stain and culture suffer from some shortcomings like poor sensitivity and the longer turnaround time, influence of prior antibiotic therapy respectively, and this led to more rapid tests being employed. Latex Agglutination Tests (LAT) were the most popular tests for their speed of identification and simplicity in manipulation. However, the success rates reported varied among various commercial kits. Tilton RC et al., found that Directigen, Bactigen and Phadebact CSF kits could detect 80%, 66% and 50% of N. meningitides, 100%, 100% and 86% of S. pneumoniae, and 78%, 100% and 83% of H. influenzae type b respectively when compared to culture [4]. Sippel JE et al., reported that 83%, 77% and 93% of the cases were due to H. influenzae, S. pneumoniae and N. meningitides using Directigen kit [5]. Use of molecular methods has profoundly improved the accuracy and speed in diagnosis. Conventional PCR in a single tube by Tzanakaki G et al., had an overall specificity of 100% and sensitivities 92.3%, 88% and 93.9% for S. pneumoniae, H. influenzae and N. meningitidis [6]. Further improvement in the technologies of molecular biology, newer tests like real time PCR and Loop Mediated Isothermal Amplification (LAMP) assays increased the detection of bacteria many folds [7,8].
The present study was undertaken with the objectives of optimizing a multiplex PCR assay for the simultaneous detection of Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis and to assess the diagnostic power of conventional tests.
Materials and Methods
A descriptive study was conducted at the Department of Microbiology with the collaboration of Departments of Medicine and Paediatrics of Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. The study was approved by Institute Research and Human Ethical Committee. A structured proforma was used to record clinical and demographic data.
Inclusion criteria: CSF samples were collected from all patients attending JIPMER hospital between November 2012 and July 2014 with clinical signs and symptoms suggestive of acute pyogenic meningitis (fever, headache, neck stiffness and altered sensorium). CSF samples with any of the following findings were included for subsequent assays:
(i) Total leucocyte count > 2000/μL;
(ii) Total neutrophil count > 1180/μL;
(iii) Glucose concentration < 34mg/dL; and
(iv) Protein concentration > 220 mg/dL [9].
Patients with clinical features suggestive of meningitis with risk factors like recent head injury or neurosurgery were excluded from the study. The study was conducted after written and informed consent was obtained from the parent/legal guardian.
Specimen collection and processing: CSF was collected in a labelled sterile vial by the clinician under aseptic conditions by a lumbar puncture, which was immediately transported to the laboratory. A few drops of the sample was placed on a clean glass slide, air dried, heat fixed, stained by Gram stain procedure and examined for pus cells and bacteria. A loopful of the sample was inoculated onto 5% sheep blood agar, chocolate agar, and MaConkey agar and the plates were incubated in 5% CO2 at 37°C for 24 to 48 hours. After incubation, bacteria were identified by their colony morphology and phenotypic tests [Table/Fig-1]. Antigen detection was performed with commercial antigen detection test kit (Latex Agglutination Test, DirectigenTM Meningitis Combo Test Kit, Becton Dickinson, USA) according to manufacturer’s instructions. Specimens were stored at -80°C for further tests.
Assays for identification of targeted organisms. The features in Gram stain, colony morphology and results of the phenotypic tests were used to identify the targeted organisms.
| Organism | Gram stain | Colony characters | Phenotypic tests |
|---|
| Streptococcus pneumoniae | Gram positive diplococci, lanceolate shape | α hemolytic colonies on blood agar, bleaching on chocolate agar | Catalase negativeOptochin sensitiveBile solubleInulin fermenter |
| Haemophilus influenzae | Gram negative cocco-bacilli with or without filamentous forms | Heavy growth nearS. aureus streak line (Satellitism)Large round, grayish smooth, convex coloniesopaque colonies on chocolate agar. | Hemin and NAD growth factor requirement |
| Neisseria meningitidis | Gram negative diplococci | Round, grayish-moist, smooth, convex, glistening colonies on blood and chocolate agar | Oxidase positiveGlucose and MaltoseFermented |
Multiplex polymerase chain reaction: DNA was extracted using commercial DNA extraction kit (QIAGEN, IN) following manufacturer’s instructions. The following genes were targeted in the assay: capsulation (bexA) in H. influenzae, capsular transport (ctrA) for N. meningitidis and pneumolysin (ply) in S. pneumoniae using published primers [6].
The reaction conditions were optimized: A total of 50 μL of the reaction mixture had 18 μL of a 2X commercial master mix (Origin Labs India), with 0.6 μM each of forward and reverse primers and 20 μL of DNA sample. Initial denaturation was done at 95oC for five minutes followed by 35 cycles of denaturation at 95oC for 25 seconds, annealing at 57.3oC for 40 seconds and extension at 72oC for one minute. The reaction was carried out in Mastercycler nexus Gradient thermal-cycler (Eppendorf, NA). Standard strains of S. pneumoniae (ATCC® 49619™), H. influenzae (ATCC® 49766™) and N. meningitidis (ATCC® 13090™) were used as positive controls in each run. The amplified DNA was separated by gel electrophoresis in 3% agarose gel (stained with ethidium bromide) and visualized under UV light (Gel doc XR system, BIO-RAD, CA). The organisms were identified based on the size of the amplicons as described previously [6].
Statistical Analysis
Any specimen which gave a positive result in any of the test procedures used was taken as positive for that particular organism. Association of various clinical features and biochemical parameters with the presence of the disease was tested using Fischer’s-exact test and a two tailed p-value < 0.05 as considered statistically significant. The sensitivity, specificity, positive predictive value, negative predictive value of the conventional tests were calculated in comparison with PCR (gold standard) at 95% confidence intervals using Graphpad QuicCalc software.
Results
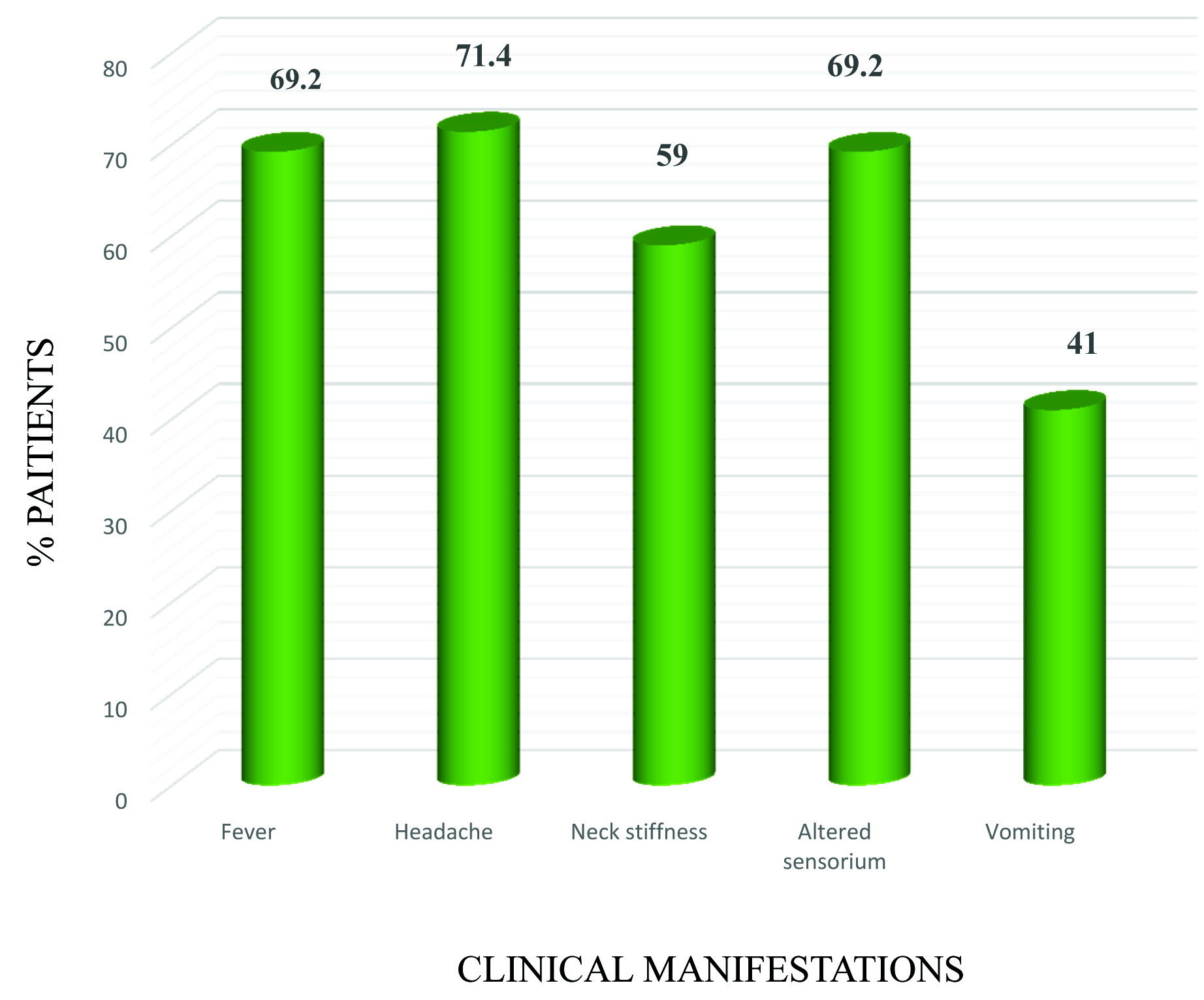
A total of 125 clinically suspected cases of acute meningitis were included, of which 39 (31.2%) were identified as acute bacterial meningitis based upon the different parameters. Age ranged from a one-day-old neonate to 72 years (mean 14.5 years) with a male to female ratio of 2:1. Majority of confirmed cases were found in patients aged more than 3 years (22/39, 56.4%) followed by 12-30 years (7/39, 17.9%) and more than 50 years (4/39, 10.25%). Clinical manifestations of the patients with confirmed bacterial meningitis (n=39) were shown in [Table/Fig-2]. A high cell count was found in only 79.5% (of which 95% showed neutrophil preponderance). The three organisms targeted by PCR reduced the glucose levels Considerably when compared with other pathogens. Protein levels increased beyond 220 mg% in 64%. Only 59% of the patients had all the three laboratory findings.
Clinical manifestations of the patients with confirmed bacterial meningitis (n=39). Percentage of the patients showing each clinical sign was represented.
Only 10 out of these 39 cases were found to be due to one of the targeted organisms while the rest had various other pathogens (11/29 were Klebsiella pneumoniae, 6/29 were Escherichia coli, 4/29 were Staphylococcus aureus, 3/29 were Enterococcus spp. and 1/29 was Streptococcus pyogenes). Non-fermenting Gram negative bacilli were isolated in the remaining four samples. The single case due to H. influenzae (positive by PCR alone) was a 14-year-old girl. N. meningitidis was detected in a 13-year-old boy, and was positive by culture, LAT and PCR.
Members of Enterobacteriaceae family were more commonly isolated in age group more than 2 months. S. pneumoniae was isolated from age group 3 month- 3 years (3/8), 12-20 years (3/8) and 21-30 years (2/8).
When compared with culture, Gram stain was positive in 16/39 (41%) of the samples (4/7 for S. pneumoniae, 4/11 for K. pneumoniae, 3/6 for E. coli, 1/4 for S. aureus). Four of the 39 cases were diagnosed as acute pyogenic meningitis based on Gram stain findings alone. The culture was positive in 33/39 (84.6%) samples.
LAT was positive in all the eight culture confirmed cases due to S. pneumoniae and N. meningitidis Multiplex PCR was positive for all the culture-confirmed cases [Table/Fig-3] and was able to detect one additional isolate each of S. pneumoniae and H. influenzae.
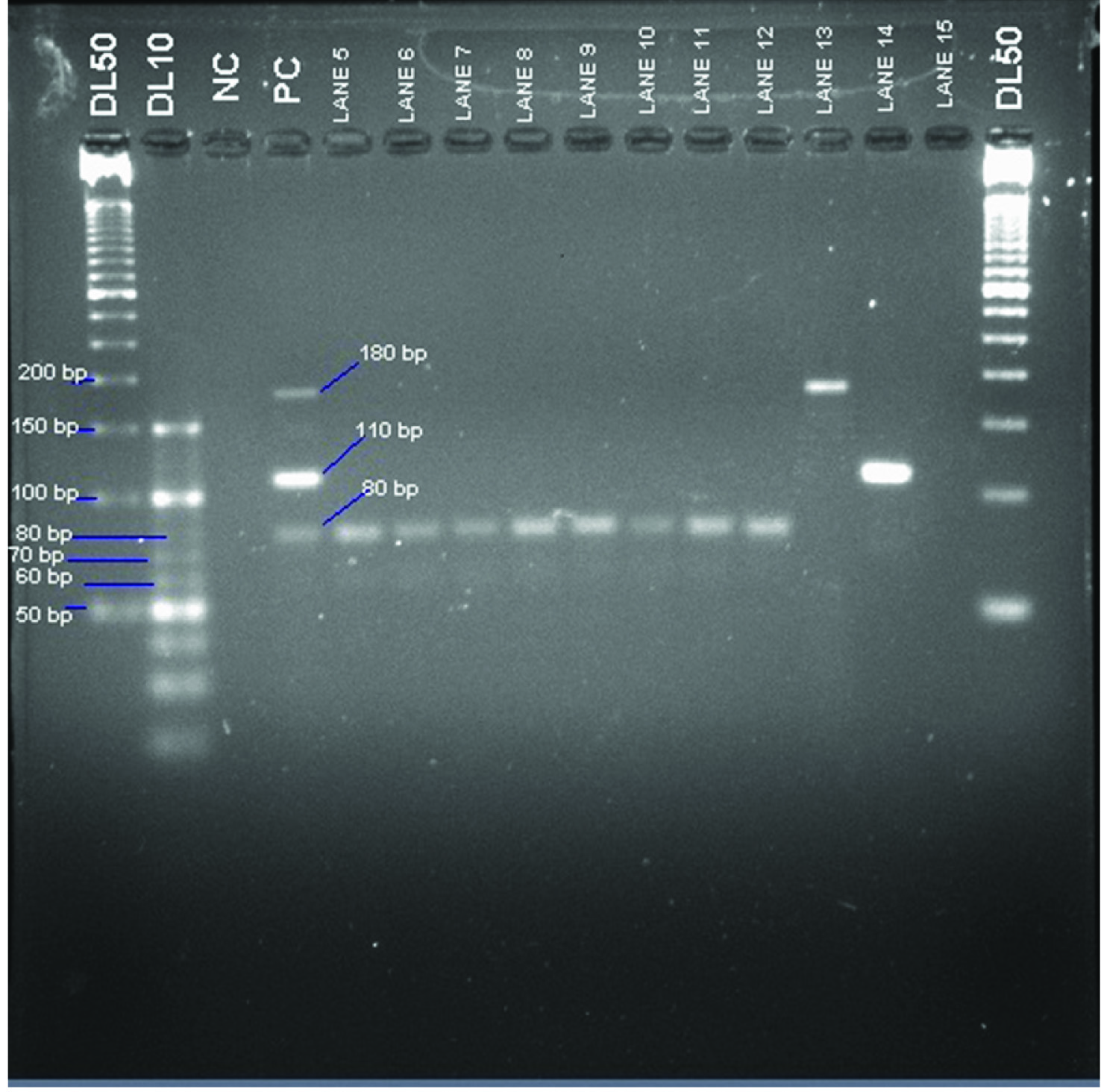
Gel electrophoresis of the samples after polymerase chain reaction; Lane 1- 50 bp molecular weight marker, Lane 2- 10 bp molecular weight marker, Lane 3 – Negative control, Lane 4- Positive controls {S. pneumoniae (80 bp), H. influenzae (180 bp) and N. meningitidis (110 bp)}, Lane 5- 12- Samples positive for Streptococcus pneumoniae, Lane 13 – Sample positive for Haemophilus influenzae, Lane 14 –Sample positive for Neisseria meningitidis, Lane 15- Sample negative for all three targeted organisms, Lane 16- 50 bp molecular weight marker.
Sensitivities, specificities, positive and negative predictive values of the different conventional tests in comparison with Multiplex PCR were calculated [Table/Fig-4]. Gram staining was found to have the least sensitivity while both culture and LAT had moderate sensitivity and excellent specificities.
Comparison of different conventional test methodologies with multiplex PCR results (n=125) for the three targeted bacteria. PPV-Positive predictive value; NPV - Negative predictive value; CI-Confidence Interval; LAT-Latex Agglutination Test.
| Test | No. positive | Sensitivity (%) (95% CI) | Specificity (%)(95% CI) | PPV (%)(95% CI) | NPV (%)(95% CI) |
|---|
| Gram stain | 4 | 40 (12.40%-73.63%) | 95.56 (89%-98.75%) | 50 (16.01%-83.99%) | 93.48 (86.34%-97.55%) |
| Culture | 8 | 80 (44.43%-96.89%) | 100 (95.94%-100%) | 100 (62.91%-100%) | 97.83 (92.35%-99.67%) |
| LAT | 8 | 80 (44.43%-96.89%) | 100 (95.94%-100%) | 100 (62.91%-100%) | 97.83 (92.35%-99.67%) |
Discussion
In the present study, rates of isolation of the three targeted organisms were found to be similar to the previous reports in India [1]. Although a vaccine against H. influenzae was introduced from December, 2011 as a part of universal immunization schedule in Tamil Nadu and Puducherry, we had only a single case of meningitis due to this organism. The low prevalence of H. influenzae b may be due to herd immunity of the vaccine administered to other children. In the present study we had a single case of Haemophilus influenzae meningitis in a 14-year-old child which is in contrast to the general observation that this organism usually infects much younger children. This shift in the susceptible age group could be due to the immunization program implemented. However, this can only be inferred by further investigations. N. meningitidis was very rare in South India (the one case in this study was only the second case reported in our hospital in the past 25 years). Similarly, low prevalence of N. meningitidis was reported in a study conducted in Bangalore (4 cases in a 10-year period).
It is evident from the present study that the aetiological agents have been changing over the past few years with the members of the family Enterobacteriaceae surpassing the traditional pathogens. Chakrabarti P et al., reported only two cases of pneumococcal meningitis and none due to H. influenzae and N. meningitidis. Acinetobacter spp., E. coli, Pseudomonas spp. were more frequently isolated from CSF samples of children [10]. A study from Taiwan reported an increased incidence of pyogenic meningitis due to Klebsiella, of which many were patients who underwent neurosurgical procedures or with head injuries and they also had underlying diseases or associated pathologies (liver abscesses, Diabetes Mellitus, etc.,) [11].
In our study, none of these patients had any underlying predisposing factors or associated conditions. Streptococcus group B and Listeria monocytogenes have been reported to be a cause of neonatal meningitis [12,13], but were not observed in our study. This can be due to low maternal genital carriage rate of group B streptococci in our study. These findings further support a similar retrospective study carried in Bangalore by Mani R et al., (neither one of the pathogens were found in a 10-year study period) [1].
In our study, 95% of the study population had presented with at least two of the four major clinical features (fever, neck stiffness, altered sensorium and headache). The clinical presentation depends on various factors like age, immuno-competence, prior antimicrobial therapy, etc. A report on paediatric meningitis had observed that young children rarely present with classical signs and symptoms, and medical attention was sought for nonspecific complaints like incessant crying, poor feeding and lethargy [14]. The characteristic maculopapular rash seen in Meningococcal meningitis was not observed in the single case in our study group.
In the present study, the findings of culture and Gram stain for the targeted organisms were discordant in the culture confirmed meningitis (n=39). Only 10.25% of the samples were positive by Gram staining, while 20.5% were culture positive. This signifies that Gram staining has poor sensitivity (40%). This was lower than the sensitivity of Gram stain (ranging from 60% to 90%) reported in other studies [15,16]. The reported culture positivity ranges between 85.3% for S. pneumoniae and 77.9% for meningococcal meningitis, while for any of the three pathogens it was 81.3% [16]. However, the single case of H. influenzae meningitis in the present study was culture negative.
Antigen detection assays have been used for more than four decades for the diagnosis of bacterial meningitis. These tests are rapid, require no special equipment, easy to perform and give results within 15 minutes. Although expensive, they have been widely employed due to the perceived usefulness in patients who had received antibiotics. But in the last two decades, the utility of these tests has been called into question with several studies reporting its limitations. Nigrovic LE et al., in a ten-year retrospective study in children who had received antibiotics before CSF sample collection could not detect any case positive by latex agglutination [17]. In another study by Perkins MD et al., out of 478 CSF samples tested, only seven samples showed positive result by latex agglutination [18]. Bronska E et al., had reported a significant decline in the sensitivity of the test in patients who had received prior antibiotics. They found a sensitivity of 60% in patients without any treatment which decreased to 22% in the treated group [19]. In the present study, we used a kit based latex agglutination test for the detection of capsular antigens of Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. The sensitivity of the latex agglutination was same as the culture at 80% as all the culture positive cases were positive by LAT. No additional cases were detected by this method. This corroborates the questionable role of antigen detection in the diagnosis of pyogenic meningitis.
Molecular assays like PCR have been designed to overcome the limitations of culture and antigen detection tests and were extensively evaluated for the diagnosis of pyogenic meningitis cases. It was shown that PCR could detect a greater number of positive cases compared to other methods as it does not depend on the viability of the organism and is not affected by a prior antibiotic administration. Though culture has the advantage of testing antibiotic sensitivity over PCR, the latter is more sensitive and rapid. Tzanakaki G et al., reported that conventional multiplex PCR had a positive predictive value of 100% and negative predictive value of 99.1% to 99.5%, where the pathogens could be detected earlier. The limit of detection of this test was 5-10 picogram of DNA [6]. Radstrom P et al., used a semi-nested PCR strategy and found a high sensitivity and specificity of 94% and 96 % respectively [20]. In our study, we employed conventional multiplex PCR and evaluated the utility of the test for simultaneous detection of primary pathogens causing bacterial meningitis. Multiplex PCR detected all the culture positive cases for the three targeted organisms exhibiting 100% sensitivity. Additional two cases, one each of Streptococcus pneumoniae and Haemophilus influenzae b were detected by PCR. A pan-bacterial PCR which detects all the bacterial pathogens in CSF without differentiating between the bacteria would have been a better test as we had encountered organisms other than the three targeted ones more commonly in our population.
Advances in molecular techniques have led to far more sensitive tests than conventional PCR such as real-time PCR and the LAMP assay. There were several published reports demonstrating the superiority of these tests over conventional tests in the diagnosis of pyogenic meningitis. Corless CE et al., evaluated the diagnostic accuracy of RT-PCR using S. pneumoniae, H. influenzae, and N. meningitidis specific primers in culture-confirmed cases and found that the sensitivity for S. pneumoniae was 91.8%, H. influenzae 100%, and N.meningitidis was 88.4%, while specificity for all organisms were 100% [21]. Several LAMP assays have been evaluated to assess the utility in detection of bacterial meningitis, and were found to be more accurate and rapid. Kim DW et al., in their study found that detection limit of LAMP assay was more than 100 times lower than that of conventional PCR method [22].
Limitation
The small quantity of CSF received in a majority of cases which precluded centrifugation of the specimen, the probable reason for the poor sensitivity of Gram staining observed in the present study. An internal control was not used in PCR reactions, the use of which would have helped us to rule out the presence of PCR inhibitors in the specimens.
Conclusion
A significant shift in the aetiological agents of acute pyogenic meningitis was observed, with members of Enterobacteriaceae replacing the three primary pathogens. Molecular techniques are significantly more effective for establishing the aetiological diagnosis of pyogenic meningitis, although they cannot completely replace conventional tests. Implementation of pneumococcal vaccination in universal immunization programs is imperative as it has been found to be the most frequently isolated pathogens for which effective vaccines are available.
Financial Support
This work was supported by Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) Intramural Research fund.