SPN is a well-circumscribed, apparently encapsulated, low-grade malignant tumour having female predilection, with highest incidence in second and third decades of life [1]. This neoplasm accounts for 0.9% to 2.7% of all exocrine pancreatic tumours, and 5% of all cystic tumours of the pancreas [1]. Previously it was classified as a tumour of uncertain malignant potential. Tumour arises from acinar cells of the exocrine glandular component of pancreas and composed of poorly cohesive monomorphic epithelial cells forming solid and pseudopapillary structures [1]. Radiologically, this sharply demarcated tumour has variable solid-cystic appearance, but without significant heterogenous contrast enhancement [2]. These tumours rarely metastasize, amenable to complete excision and have excellent prognosis. Being an uncommon tumour, secondary to detection after clinical examination or incidental detection during radiological screening for some other condition, this abdominal mass needs to be completely evaluated by triphasic contrast enhanced CT scan and FNAC before surgical exploration. This neoplasm should be included in the radiological differential diagnosis of well-defined, solid or solid-cystic pancreatic mass lesions because of it’s low grade malignant character. Therefore, the study was carried out to summate the imaging and pathological features of seven cases of SPN, a relatively uncommon tumour, managed in our institute in the last three years.
Materials and Methods
The retrospective study was conducted in Department of Radiodiagnosis and Imaging in collaboration with Department of Pathology, Kasturba Medical College, Manipal University, Mangaluru, Karnataka, India, after taking Institutional Ethical Clearance. Seven cases of histologically confirmed SPN during the three years period from January 2013 to January 2016 were included in the study. These patients had undergone triphasic Multi Detector Computed Tomogram (MDCT) evaluation on a 16-slice scanner and the images were retrieved from the radiology department archives. Arterial (25 seconds), venous (60 seconds) and delayed venous (90 seconds) phases of the abdomen following the administration of 100 ml of iodinated contrast (flow rate of 2.5 ml/second) was performed in all the cases. All axial and angiographic images, as well as coronally and sagittally reformed images were reviewed. The MDCT was reviewed by two radiologists and they were not blinded to the final histopathological diagnosis. This was followed by Computer Tomogram (CT) guided Fine Needle Aspiration (FNA) of the pancreatic lesions and subsequent surgical removal. A 22-guage needle was used for performing the CT-guided FNAC under local anaesthesia. The diagnosis of SPN was established on the FNA cytology followed by histopathological examination. Entirely cystic lesions with previous history of acute pancreatitis and cases with elevated serum tumour markers were excluded from the study.
Source images with the body soft-tissue algorithm, multiplanar reformatted and 3D-images were analysed. The CT features evaluated were: dimensions of the mass, site (head, neck, uncinate process, body, tail), largest diameter of the lesion, attenuation, well or ill-defined borders, capsulation, presence of calcification within the lesion, and contrast enhancement pattern. Other features which were examined included mass effect, infiltration into other neighbouring organs, vascular involvement, lymph node involvement, and metastasis.
Statistical Analysis
Since there were only seven cases, statistics was represented as percentages alone.
Results
All the seven cases in this study, were female patients with an age range of 13-35 years (mean: 23.3 years). The tumour was located in the tail of pancreas in four patients, followed by one case each in the body, head and neck of pancreas. The size of the tumours varied from 2.5-14 cm (mean: 5.3 cm) in maximum axial dimension [Table/Fig-1], and they were round to oval in shape as well as encapsulated.
Summary of radiological and pathological findings.
| Case no. | Age (years) | Location | Size (cm.) | CT findings | Histological findings |
|---|
| 1. | 29 | Tail | 4.0 | Well-defined hypodense lesion | Uniform cuboidal cells arranged around delicate fibrovascular core, myxoid change in stroma, nuclear grooving |
| 2. | 13 | Tail | 2.5 | Peripherally enhancing predominantly cystic | Uniform cuboidal cells arranged around delicate fibrovascular core, stromal hyalinization and myxoid change, oncocytic cells, nuclear grooving, secondary changes |
| 3. | 22 | Tail | 6.0 | Moderately enhancing hypodense and cystic lesion with peripheral microcalcification ([Table/Fig-3a-d]) | Uniform cuboidal cells arranged around delicate fibrovascular core, stromal hyalinization, nuclear grooving, secondary changes |
| 4. | 20 | Body | 4.5 | Well-defined hypodense lesion | Uniform cuboidal cells arranged around delicate fibrovascular core, oncocytic cells, nuclear grooving, secondary changes |
| 5. | 35 | Head | 14 | Heterogeneously enhancing solid lesion with peripheral microcalcifications. ([Table/Fig-2a-d) | Uniform cuboidal cells arranged around delicate fibrovascular core, stromal hyalinization, clear cells, nuclear grooving, secondary changes |
| 6. | 29 | Neck | 3.0 | Well-defined hypodense lesion ([Table/Fig-3e,f]) | Uniform cuboidal cells arranged around delicate fibrovascular core, stromal hyalinization, clear cells, nuclear grooving, secondary changes |
| 7. | 15 | Tail | 3.0 | Well-defined hypodense lesion | Uniform cuboidal cells arranged around delicate fibrovascular core, stromal hyalinization, oncocytic cells, nuclear grooving, secondary changes |
On CT scan review, five patients showed a well-defined mass with heterogeneous attenuation with solid and cystic components with displacement of adjacent structures. All lesions had 25-50 HU (pre-contrast) solid/soft tissue attenuation. Only in one patient, few hyperdense foci of 55-60 HU in plain scan, represents peripheral soft calcification {Case 3: [Table/Fig-2a,b]}. Foci of peripheral calcifications were noted in two cases {Case 3,5: [Table/Fig-1]}. All the solid enhancing portions showed moderate enhancement of at least 20-30 HU compared to unenhanced scan, on the other hand the cystic parts remained unenhanced with <5 HU variation in comparison to the plain scan. In two patients tumour was predominantly solid and enhancing and size =3 cm {Case 6,7: [Table/Fig-1]}. Of the seven patients, five had heterogeneous enhancement with large non-enhancing central areas. Two patients with smaller tumours had predominantly solid enhancement pattern and few non-enhancing punctuate foci. In five cases there was no vascular involvement. In one case, the mass {[Table/Fig-2a]: asterix} was significantly compressing Inferior Vena Cava (IVC) {[Table/Fig-2b,d]: arrow}, however there was no IVC invasion or thrombosis. Microcalcification was also noted in the periphery of the tumour (Case 3) {[Table/Fig-2c,d]: arrowhead}. Similarly, Case 5 [Table/Fig-3a-d] also showed peripheral microcalcification {[Table/Fig-3a]: arrow}. Predominantly solid tumour (Case 6) is represented by hypodensity on CT scan [Table/Fig-3e,f]. In three cases, portal vein was compressed and displaced, and in one case the portal vein was partially encased for less than 180° circumference. One case of tumour in the pancreatic tail was closely abutting the splenic vein. Radiological diagnoses on triphasic CECT was that of a well-defined solid-cystic and solid neoplasm in two and five cases, respectively, with SPN and cystadneomas as a differential diagnosis.
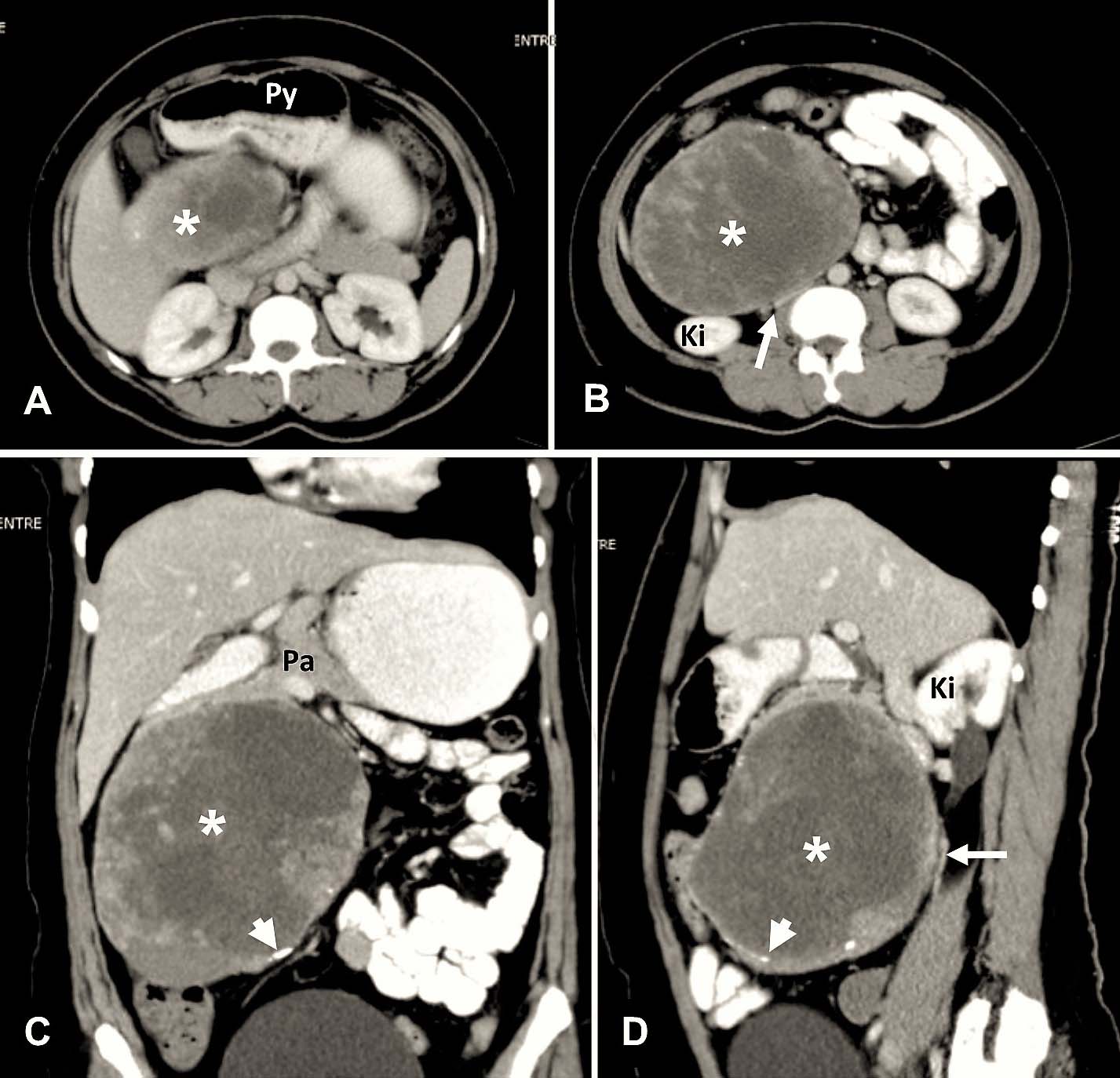
Case 3: a,b) Axial section at the level of superior aspect of head of pancreas shows a well-defined, heterogeneously enhancing mass (asterix) with peripheral microcalcifications (white arrowhead), displacing the pylorus (Py) of stomach anteriorly. The tumour (asterix) is displacing the bowel loops peripherally. IVC (white arrow) is compressed with maintained fat planes; c) Coronal section shows the tumour (asterix) is arising from the region of head of pancreas (Pa); d) Sagittal section shows the IVC (white arrow) and right kidney (Ki) displaced posteriorly.
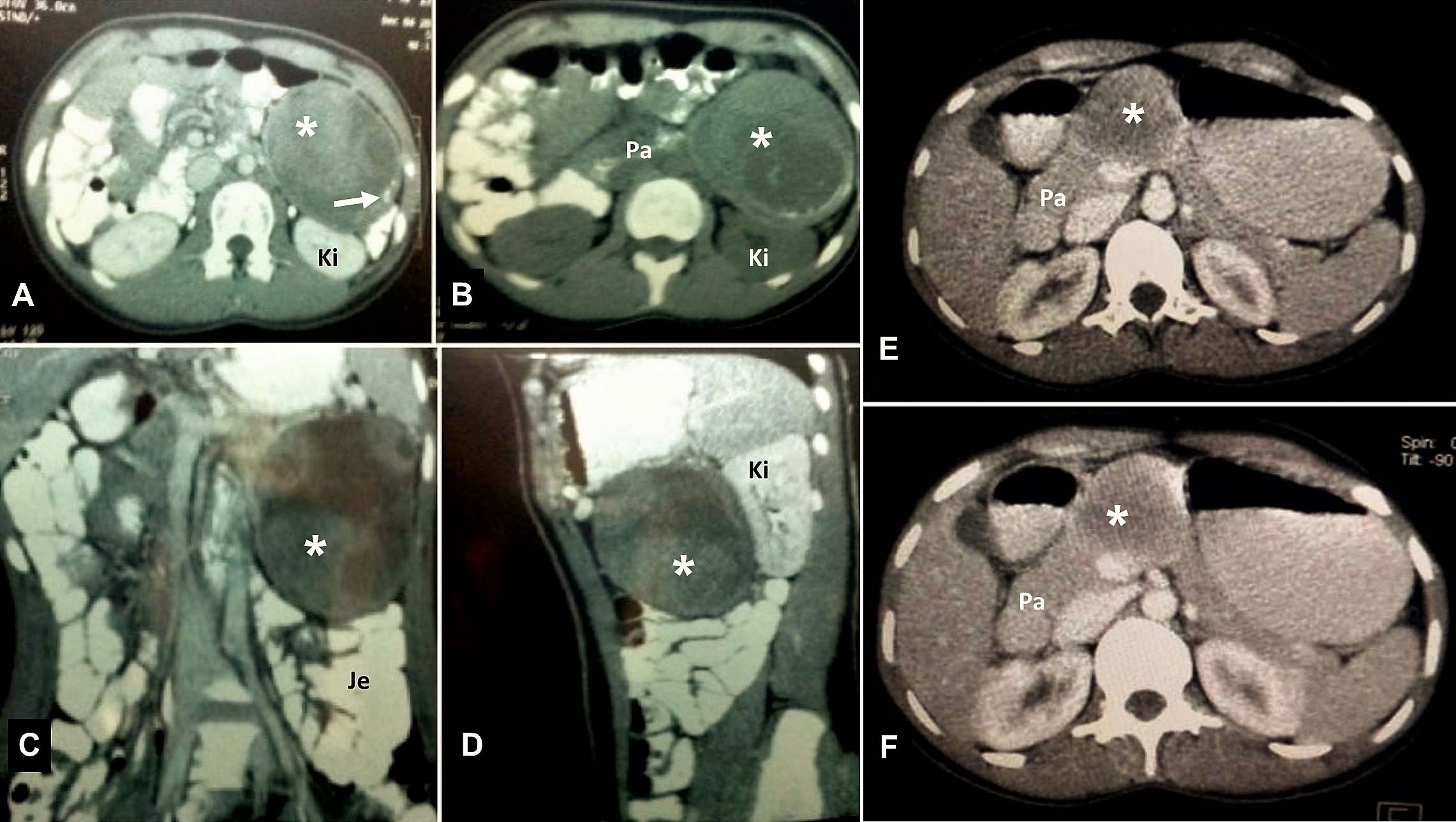
Case 5 – Axial Contrast (a) and Plain (b) sections show a large heterogeneous mass (asterix) lesion involving the tail of the pancreas with minor peripheral soft calcifications (white arrow). The lesion (asterix) indenting the anterior surface of the kidney (Ki) and displacing the jejunal (Je) loops is seen on the coronal (c) and sagittal (d) sections. Case 6 – Axial sections (e & f) with contrast shows a well-defined, minimally enhancing hypodense lesion (asterix) in the body and neck of the pancreas (Pa).
Grossly, all the lesions were nodular masses. Except Case 3, all tumours on cut section were predominantly solid, pale to dark brown, heterogenous cut-surface with haemorrhage and necrosis, and soft to firm in consistency [Table/Fig-4].
Well-defined tumour in tail of pancreas with attached spleen. Cut surface of tumour is solid, pale to dark brown, heterogeneous with haemorrhage and necrosis, and soft to firm in consistency.

Fine needle aspiration revealed cellular smears comprised of cuboidal, medium-sized cells arranged in small loosely cohesive groups, microacini and singly dispersed pattern. Thin, delicate to thick hyalinised branching fibrovascular papillae {arrow; [Table/Fig-5a]} lined by one or more layers of monotonous tumour cells having indistinct cell borders, were characteristically noted. The uniform nuclei of tumour cells were round or oval in shape and had finely dispersed chromatin, inconspicuous nucleoli and occasional intranuclear grooves [Table/Fig-5b]. The smear background exhibited necrotic debris, macrophages, cholesterol crystals and haemorrhage. Intra- and extracytoplasmic metachromatic hyaline globules were also observed.
a) Branching papillary fragment with slender fibrovascular core (arrow; Papanicolaou, x40); b) Uniform round to oval nuclei with grooves (Papanicolaou, x400). Tumour composed of poorly cohesive, uniform, cuboidal cells arranged around delicate fibrovascular core appearing as papillae; c) H&E x40; d) H&E x200); e) The tumour cells had round to oval nuclei and moderate amount of eosinophilic cytoplasm (H&E x200); f) Hyalinization and myxoid change in the core of the pseudopapillae (H&E x40); g) Oncocytic cells, nuclear grooves and few hyaline globules (H&E x400).
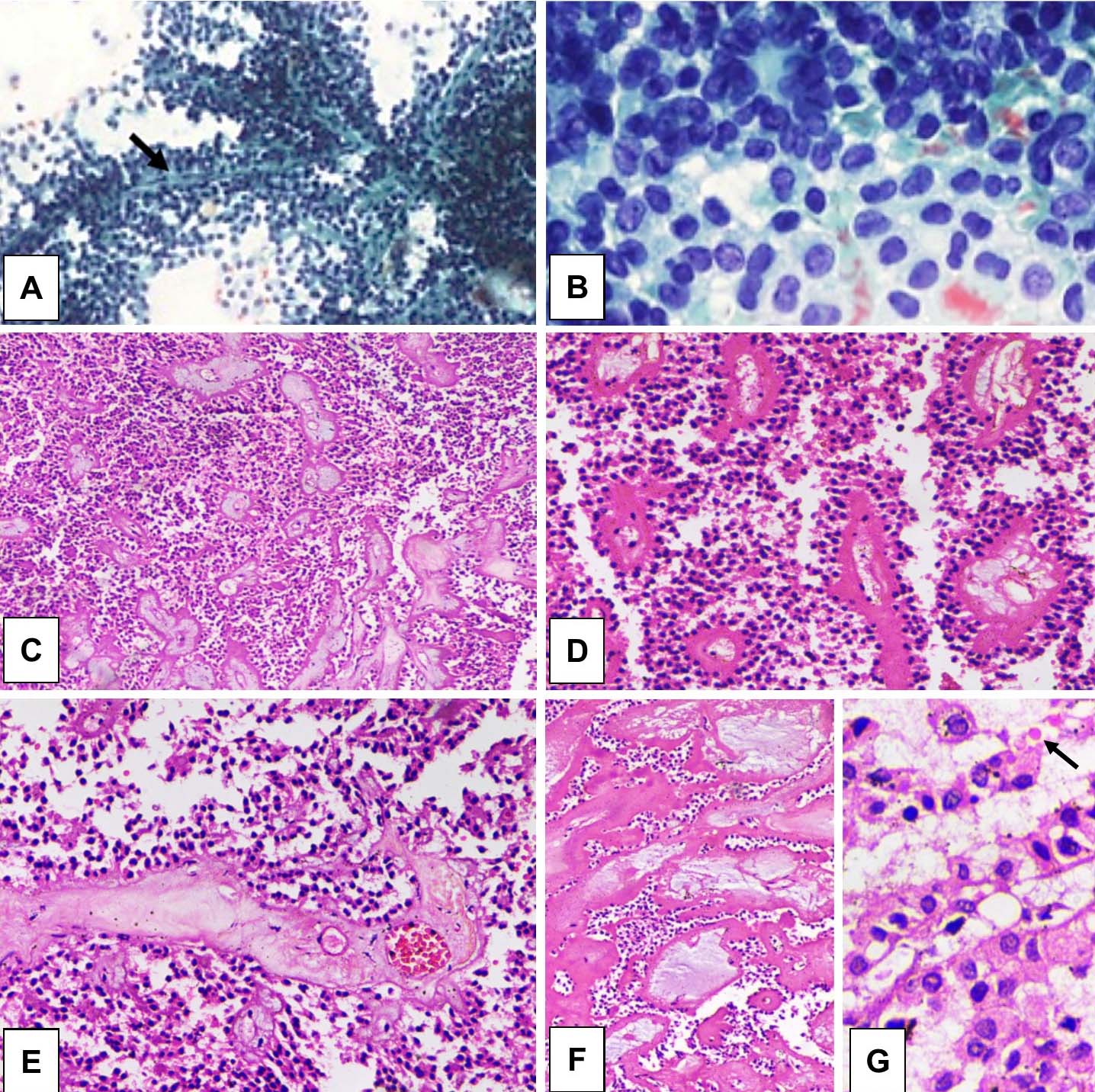
On histopathological examination, the tumour was composed of poorly cohesive [Table/Fig-5c], uniform, cuboidal cells arranged around delicate fibrovascular core [Table/Fig-5d]. The tumour cells had round to oval nuclei, fine chromatin and moderate amount of eosinophilic cytoplasm [Table/Fig-5e]. The characteristic features like nuclear grooves were seen in all the cases. Stromal changes such as hyalinization and myxoid change in the core of the pseudopapillae [Table/Fig-5f] were observed in 5 and 4 (out of 7) cases, respectively. Oncocytic cells [Table/Fig-5g] and clear cell change was observed in 3 and 2 (out of 7) cases, respectively. Secondary changes like, scattered intra- and extracellular hyaline globules {arrow; [Table/Fig-5g]}, cholesterol clefts, giant cells, foamy histiocytes and moderate chronic inflammatory infiltrate were noted in all cases. Histology did not reveal any feature of parenchymal, perineurial or angioinvasion. Review of patient records revealed that three out seven cases are on follow up for last two years and are clinically stable without any evidence of recurrence or metastasis.
Discussion
Solid pseudopapillary neoplasms are slow growing exocrine pancreatic tumours, thought to arise from acinar cells of pancreas and are biologically different from pancreatic adenocarcinoma. This tumour was first described by Frantz VK in 1959 [3] as a “papillary tumour of the pancreas, benign or malignant” has a strong predilection for adult females with a male:female ratio of 1:10 [2]. All the seven cases in this study were female patients and similar exclusivity in females was reported by Patil TB et al., [4], and other studies have also consistently reported female preponderance [5-7]. It is more common in young non-Caucasian women, usually Asian and African-American women, between the second and third decades of life [8]. The mean age of 23.3 years in the present study was concordant with the studies of Patil TB et al., and Mao C et al., [4,6]. Though, SPN can occur in any part of the pancreas, but tail of the pancreas appears to be the most common location. In this study, in 57.1% cases the tumour was located in tail of pancreas, as were 50% and 71% cases reported by Patil TB et al., and Huang HL et al., respectively [4,5]. The average size of 5.3 cm in the present study was close to the mean size of 5.8 cm reported by Wang DB et al., [9]. Other researchers have reported higher average size of approximately 10 cm [5,6]. In the series by Paruchuri RK et al., all nine tumours were encapsulated and calcification was seen in two out of nine cases, similar to our experience [7].
The purpose of differentiating this low grade malignancy from an overtly malignant adenocarinoma, is to decide on the extent of surgery required to safely remove the tumour without recurrence [10]. This tumour has an indolent course and prognosis is good after complete resection, as SPNs are usually confined within the pancreas. It compresses or displaces adjacent structures and can locally infiltrate or metastasize rarely [11,12]. In a study conducted by Wang DB et al, the size of the tumour has been shown to correlate with capsular invasion. A size greater than 6 cm was shown to have more association with capsular invasion [9]. Liver has been described as the most common site for metastasis. Lymph nodal metastasis may be seen in left gastric, splenic, retropancreatic, celiac, and mesenteric lymph nodes. SPNs demonstrating angioinvasion, nerve sheath invasion or lymph node and liver metastases are usually seen in older age with a male predilection and are categorised as solid-pseudopapillary carcinomas by WHO classification [1,2,8].
On X-ray they appear as large masses, with occasional calcifications, displacing adjacent structures like stomach or bowel loops [13]. Ultrasound shows a well-defined mass with solid and cystic components and increased vascularity. Contrast enhanced CT shows an encapsulated lesion with enhancing solid and non-enhancing cystic areas with some showing calcific foci. Haemorrhagic density may be seen within the lesion. Lesions smaller than 3 cm may present as solid lesions. Solid pseudopapillary neoplasms may sometimes grow to large sizes with a mean diameter ranging from 6 cm to 10 cm [10]. On MDCT the SPN are encapsulated tumours, which are round to oval in shape and exhibit heterogeneous attenuation with peripheral iso- to hyperdense areas [7], similar to our study. In a study of 24 cases, by Wang DB et al., described capsular disruption in 37.5% cases, followed by infiltration of adjacent organs that is spleen, duodenum and stomach in 25% cases, nodal and liver metastases (12.5%) [9]. Further pancreatic invasion (12.5%), nearby vascular involvement (8.3%), pancreatic duct dilatation (4.16%), peripancreatic fat invasion (4.16%) was also reported [9]. Rare presentation in male patients and atypical manifestations include metastasis, ductal obstruction, extracapsular and pancreatic invasion, simulation of islet cell tumour or intratumoural extensive calcification [14]. However, we did not come across any case with overt malignant features.
On gadolinium enhanced Magnetic Resonance Imaging (MRI), the enhancement pattern is similar to that seen on CT. However, MRI is better at demonstration of haemorrhagic component of the lesion [15]. MRI typically demonstrates a well-defined lesion with heterogeneous mixed T1 and T2 signal intensities with areas of high signal intensity on T1-weighted images and low or inhomogeneous signal intensity on T2-weighted images corresponding to haemorrhagic areas [8]. None of the radiological features are characteristic of SPN and these features can also be seen in other benign and malignant tumours of pancreas. However, a young female having a well-circumscribed and capsulated tumour in the tail of pancreas with haemorrhage and cystic changes, appearing as heterogeneously cystic central component and solid periphery, is in all likelihood SPN.
Grossly SPN may mimic pancreatic pseudocyst or other cystic neoplasms. Yellow or haemorrhagic cut surface of SPN separates it from other cystic pancreatic neoplasms. Microscopically pancreatic pseudocyst lacks epithelial lining [1]. Differential diagnosis for SPN would include pancreatic cancer and pancreatic Neuroendocrine Tumours (NET) [10]. Hyperattenuation of SPN compared to the surrounding pancreatic parenchyma on contrast-enhanced triphasic CT images is due to SPN’s rich blood supply, which helps to differentiate it from pancreatic neuroendocrine tumour. However, small atypical SPNs are usually hypoattenuating and become slightly hypo- or iso- attenuating during the portal venous phase. Also unlike adenocarcinomas, secondary changes in the pancreas, such as dilatation of the upstream pancreatic duct or pancreatic parenchymal atrophy are usually not seen in SPN. MRI also helps in differentiate SPNs from islet cell tumours, in whom cystic components have moderately increased signal intensity on T1-weighted and increased signal intensity on T2-weighted images [10].
Solid pseudo-papillary neoplasms have fairly specific histological features of SPN, such as pseudopapillae or structures resembling ependymal rosettes, but lacking acinar, cribriform and nested or trabecular pattern, which is seen in Acinar Cell Carcinoma (ACC) and NET, respectively. Nuclear grooving is a characteristic finding in SPN as opposed to salt and pepper chromatin of NET, and nuclear pleomorphism with single, central prominent nucleolus in ACC. Immunopositivity for CD10, vimentin and β-catenin, and negativity to chromogranin A (characteristic in NET), and trypsin or chymotrypsin (characteristic of ACC), further confirms the diagnosis of SPN [1]. Marchegiani G et al., reviewed 131 cases and considered 16 (12.2%) cases as malignant SPN due to the presence of at least two of the three histological features that is invasion of pancreatic parenchyma, perineural and or blood vessels. After a median of 62 months after surgery, only two (1.5%) malignant SPNs had a recurrence [16].
Mixed T1- and T2-signal intensities and progressive heterogenous enhancement pattern on MRI are important features of SPN [17]. Distal pancreatectomy with splenectomy, pylorus preserving pancreatoduodenectomy or mid-pancreatic resection can be performed depending on location of SPN by CT scan. In cases where the tumour is localised in the head of the pancreas with no invasion of adjacent structures or obstruction of pancreaticobiliary tree, a local tumour enucleation is possible. However, common bile duct and pancreatic duct dilation or local infiltration on triphasic CECT scan, may require duodenum-preserving pancreatic head resection or Whipple’s surgery, respectively [18]. Minimally invasive surgical management, that is laparoscopic excision of SPN is becoming popular, and can be performed safely with outcomes comparable to laparotomy [19].
Preoperative CT-guided FNAC and or peroperative reconfirmation of diagnosis on examination of frozen section is imperative, as surgery to remove SPN is usually less aggressive than surgical resection for adenocarcinomas. Surgical excision alone ensures excellent prognosis [18,20]. Combination of surgical resection and chemotherapy may prolong survival in malignant cases [20]. Age, primary tumour diameter, extrahepatic metastasis and complete resection of metastases and recurrence are prognostic factors of SPN [21].
Limitation
This retrospective study was limited by small sample size and lack of clinical follow-up in all cases. Furthermore endoscopic ultrasound and magnetic resonance imaging was not done for these cases.
Conclusion
Triphasic CECT most importantly provides information on local invasion and vessel involvement by SPN, which is further supplemented by preoperative diagnosis by CT-guided FNAC.