Teeth and bones are often the only available sources of DNA when human remains are extremely damaged or degraded [1,2]. Teeth are better sources of DNA when compared to bones as they are mainly protected from environmental conditions because of their peculiar composition and position within the jaw bones [2]. To the best of our knowledge, there are no evident studies on extraction of mtDNA from burnt teeth exposed to environmental conditions. Hence, bearing in mind the number of fire accidents increasing consistently propelled us to structure a study to isolate DNA from burnt teeth exposed to environment.
Materials and Methods
This in vitro study was carried out in the Department of Oral Medicine and Maxillofacial Radiology, SVS Institute of Dental Sciences, Mahabubnagar, Telengana, India, during a period of one year i.e. from December 2015 to November 2016. Sample of 20 extracted teeth were collected from the Department of Oral and Maxillofacial Surgery in SVS Institute of dental sciences. These teeth samples were incinerated in a ceramic furnace at 700°C for 15-20 minutes and randomly divided into two groups of 10 samples each. Each group sample at different times were sent to Bio Axis DNA Research Centre (located at Hyderabad, India) where extraction of DNA was carried out.
The teeth samples were kept in the soil for exposure to environmental conditions for a period of six months (Group 1) and 12 months (Group 2). These were thus subjected to the natural climatic variations of a tropical country such as winter, summer and rainy seasons. These seasons represents variation in temperatures ranging from 10°C-25°C during winter, 40°C-50°C during summer and moderate rainfall during rainy season.
The study was carried out by crushing the teeth using mortar and pestle. A 700 μl of lysis buffer {10 mM Tris, pH 8.0, 100 mM NaCl, 50 mM EDTA, pH 8.0, 0.5% S DS pH 8.0 and 20 μl proteinase K (20 mg/mL)} was mixed to 0.2 g of sample, vortexed, and incubated at 56°C, overnight. Next, 720 μl of extraction buffer (phenol: chloroform: Isoamyl alcohol of 25:24:1 ratio) was mixed to the sample, vortexed, centrifuged for 2 minutes at 15000 rpm and upper aqueous layer was collected into a sterile microcentrifuge tube. This procedure was repeated twice. Later, 720 μl of isobutanol was mixed with the aqueous layer, vortexed, and centrifuged (2 minutes) at 15000 rpm.
Later, the lower aqueous layer was relocated to the receptacle chamber of a Centricon-100 concentrator (Millipore). A 1 ml of Tris-EDTA buffer was added into the same receptacle chamber for scouring pursued by 20 minutes centrifuge at 3000 rpm. The scouring was reiterated twice till the sample got gyrated [3]. Finally, mtDNA sample was collected from the receptacle chamber into a sterile microcentrifuge tube to run on 1.2% agarose gel electrophoresis [1].
Agarose Gel Electrophoresis
To acquire a pellucid solution, 40 ml of 1.2% prepared agarose gel was placed in 800V microwave oven level for 2 minutes. This agarose solution was cooled at room temperature and 5 μl of ethidium bromide was dissolved in it. A 70% ethanol was handled to cleanse the chamber, combs and the gel casting tray and cello tape was used to seal the tray frontier carefully.
The comb is positioned in a tray containing agarose gel for about 20-30 minutes to consolidate. Followed by solidification process, the comb and tape were removed. Loading sample was prepared by mixing 10 μl of the extracted DNA and 5 μl of loading dye. After removal of the comb, the samples were loaded in the corresponding wells. The gel was allowed to run for 45 minutes to 1 hour at 100V power [1].
Gel pictures were obtained with the help of Alpha Imager mini Gel Documentation System.
Statistical Analysis
The statistical analysis was done using SPSS version 16.0. Comparison was done within the groups using single proportion test and between the two groups by two proportion test.
Results
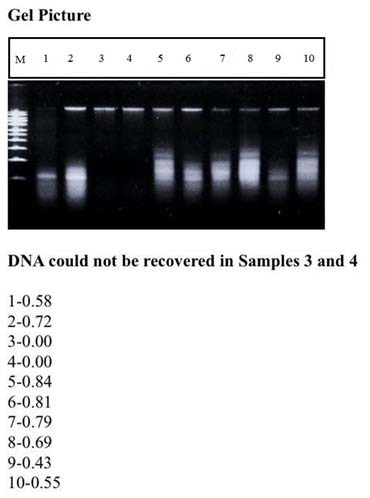
The present study showed that DNA was obtained from eight extracted teeth, six extracted teeth samples in Group 1 [Table/Fig-1] and Group 2 [Table/Fig-2] respectively.
Amount of mtDNA obtained in Group 1.
Amount of mtDNA obtained in Group 2.

The amount of mtDNA obtained among Group 1 was 0.58, 0.72, 0.00, 0.00, 0.84, 0.81, 0.79, 0.69, 0.43, 0.55 μg/ml. In Group 2, it was 0.45, 0.72, 0.00, 0.00, 0.00, 0.63, 0.72, 0.59, 0.98, 0.00 μg/ml respectively. Intragroup analysis was performed using single proportion test. (Group 1 p-value = 0.058; Group 2 p-value = 0.527). Gel pictures revealed DNA which could not be recovered from samples 3 and 4 in Group 1 and from samples 3, 4, 5 and 10 in Group 2.
Statistically significant difference was not found when both groups were compared using two proportions test [Table/Fig-3].
DNA parameters: statistical analysis using two proportion test.
| Groups | DNA test detected | DNA test not detected | Total | p-value |
|---|
| 1 | 8 | 2 | 10 | 0.342 |
| 2 | 6 | 4 | 10 |
Discussion
Disasters may occur as nature’s fury including earthquakes, floods, wildfires, hurricanes, or tornadoes. In addition, they may be manmade, directed by people through mishap or neglect, such as work, road accidents or fire or by deliberate intention, as with terrorism [1]. Forensic dentistry plays an important role in identification of an individual who is a victim of mass disaster, especially when there is a little remaining material for visible identification [2].
The revolution of DNA in 1953 by Watson JD and Crick FH prompted an upset in about all fields of logical review [4]. Jeffreys AJ et al., invented DNA fingerprints which are specific for each individual, more reliable [5,6]. They are accepted in the courts for investigation of human identification regarding sex, ethnicity and physical characteristics as legal proofs [6].
Human identification in the field of forensic sciences aims at establishing persons individuality based of DNA. Isolation of DNA is possible from all human body tissues with variations in quantity and quality of DNA extracted from the tissues like bone, saliva, blood, hair follicle, biopsy specimen, etc., [7].
The only used sources of DNA available for identification of degraded or fragmented human remains are teeth and bones [8] which led to the forensic odontology to evolve.
Teeth are better skeletal source of DNA for various reasons. As enamel has higher acellular content, it acts as a protective physical barrier from external environment [8]. As they are less prone to contamination, they have superior quality of DNA than bones [2]. Few studies support the reliability of teeth as a source of DNA for genetic analysis in human identification [1,2,8,9].
The two types of DNA employed in forensic sciences are nuclear DNA (genomic DNA) and mtDNA. In human body, nucleus of each cell consists of genomic DNA and corresponds to a DNA source for most forensic applications [10]. The mtDNA is another type of material that has high sequence variability, high number of copies per cell [7] and maternity inheritance which acts as a substantial tool [6].
Both nuclear and mtDNA effectively obtained from human skeletal stay hundreds or thousands of years after death, a time allotment far surpassing measurable necessities (as often as possible under 100 years) [2]. Over time DNA in a cell will degrade and in many situations even PCR-based testing of nuclear DNA will no longer be successful. In these circumstances, forensic scientists often turn to mtDNA analysis [11]. Chances of acquiring mtDNA are greater than that of any marker in genomic DNA even in minute skeletonized tissues [10,12]. The mtDNA analysis has become the choice for many anthropologists working on ancient skeletal material [11]. In some situations like fire accidents, bone may be degraded but still tooth remains intact and exposed to environment. Hence, the present study aimed to obtain mtDNA from burnt teeth under natural climatic conditions.
Dentin and pulp are rich sources of DNA in tooth. Dentin and cementum of tooth are rich in mtDNA [10]. The reason for obtaining mtDNA in teeth exposed to even higher temperatures could be due the presence of mtDNA in more amount and more resistance to decomposition (mtDNA degrades at a slower rate when compared to genomic DNA) [1]. Hence, even in missing cases and distant relatives cases mtDNA is beneficial [1].
Genomic DNA and mtDNA can be obtained upto 300°C and 700°C respectively [1]. Hence, the present study was aimed to obtain mtDNA from teeth subjected to 700°C.
Out of the 20 samples taken for study, DNA could be extracted from eight samples in Group 1 and six samples in Group 2. Statistical analysis was performed using two proportions test. Statistically significant difference was not found when both groups were compared. This infers that we can isolate DNA from burnt teeth exposed to environmental conditions [13].
Limitation
This study has a limited sample size and thus cannot give strong evidence. Also, the present study was carried out for a period of one year, so further long term research is required to support this hypothesis.
Conclusion
Forensic dentistry evinced an importance in the field of research for human identification using distinctive features of the teeth and jaws where mtDNA from teeth gives a reliable source of genetic information. This study elicits acquiring mtDNA from teeth even in cases where the specimens are highly decomposed and degraded due to varied environmental conditions.