Primary Intestinal Lymphangiectasia (Waldmann’s Disease) Presenting with Chylous Effusions in a 15-Year-Old
Vijay Surampalli1, Srinath Ramaswamy2, Deepanjali Surendran3, Chanaveerappa Bammigatti4, Rathinam Palamalai Swaminathan5
1 Junior Resident, Department of Medicine, JIPMER, Puducherry, India.
2 Junior Resident, Department of Medicine, JIPMER, Puducherry, India.
3 Associate Professor, Department of Medicine, JIPMER, Puducherry, India.
4 Additional Professor, Department of Medicine, JIPMER, Puducherry, India.
5 Professor, Department of Medicine, JIPMER, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Chanaveerappa Bammigatti, Additional Professor, Department of Medicine, JIPMER, Puducherry-605006, India.
E-mail: bammigatti@gmail.com
Primary Intestinal Lymphangiectasia (PIL) is a rare disease of unknown aetiology which presents in the paediatric age group with anasarca, diarrhoea, hypoproteinaemia, lymphoedema and chylous effusions. Tuberculosis, filariasis, chest trauma, malignancies and haematological disorders usually contribute to most cases of secondary lymphangiectasia and chylous effusions. We hereby describe a case of PIL presenting with chylous effusions which remained undiagnosed for eight years.
Fluid accumulation, Hypoproteinaemia, Idiopathic, Lymphatic dilatation, Milky lymph
Case Report
A 15-year-old boy was referred to Department of Medicine for the evaluation of ascites requiring repeated paracentesis. He was symptomatic for the last eight years with swelling of legs, abdominal distension, facial puffiness and breathlessness. For these complaints, he was evaluated in the local hospital but no cause was identified; he was prescribed furosemide by physician intermittently which gave him temporary relief from symptoms. He did not complain of chronic diarrhoea, decreased urine output or recurrent infections, although poor weight gain was notable since symptom onset. There was no significant family history.
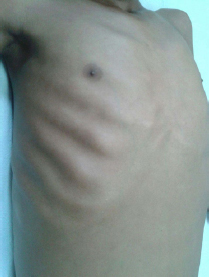
General physical examination revealed facial puffiness, bipedal pitting oedema, abdominal distension and rachitic rosary [Table/Fig-1]. Height was 155 cm, weight 36 kg and Body Mass Index (BMI) of 14.9 kg/m2 suggestive of severe wasting. Respiratory system examination revealed dullness to percussion, decreased air entry in all areas of the left hemithorax and similar findings were observed in the basal areas of the right hemithorax. Cardiovascular examination revealed muffled heart sounds. Tense ascites was found on abdominal examination.
Rachitic rosary due to vitamin D deficiency
Initial X-ray and ultrasound imaging showed massive pleural effusion on the right side, a mild pleural effusion on the left and massive ascites. Electrocardiogram showed low voltage complexes and echocardiogram revealed minimal pericardial effusion. Thoracic and abdominal paracentesis revealed milky white fluid, suggestive of chylous effusions. Triglyceride rich exudative effusion was found on analysis, confirming the same. Liver function tests showed low serum total protein and albumin and mildly elevated alkaline phosphatase. Thyroid Stimulating Hormone (TSH) was mildly elevated, Very Low Density Lipoprotein (VLDL) and High Density Lipoprotein (HDL) levels were low, 25-hydroxy vitamin D level was low and intact Parathyroid Hormone (iPTH) level was high.
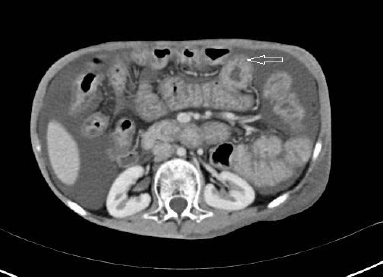
Adenosine deaminase levels were in the normal range in ascitic and pleural fluid. Smear for acid fast bacilli, mycobacterial culture and Polymerase Chain Reaction (PCR) for Mycobacterium tuberculosis were negative. Effusions were negative for malignant cells. Filarial serology, Antinuclear Antibody (ANA) and Human Immunodeficiency Virus (HIV) testings were also negative. A contrast enhanced Computed Tomography (CT) scan of the thorax and abdomen revealed ascites, diffuse bowel wall enhancement and the ‘halo sign’, characterized by inner ring of low attenuation surrounded by outer ring of high attenuation [Table/Fig-2].
Contrast CT abdomen showing the ‘halo sign’ (white arrow shows halo sign- diffuse bowel wall thickening and high attenuation around an inner area of low attenuation).
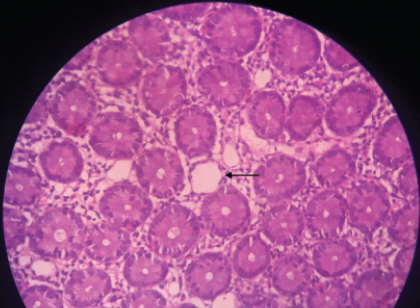
Lymphoscintigraphy [Table/Fig-3] showed no abnormal anatomy or leakage from lymphatics. Normal appearing mucosa was found on upper GI endoscopy but duodenal biopsy revealed dilated lymphatics in the submucosa with a lack of inflammatory infiltrate. Crypt architecture was preserved and Periodic Acid–Schiff (PAS) positive macrophages were not detected [Table/Fig-4]. The above findings confirmed a diagnosis of PIL.
Lymphoscintigraphy showing normal tracer uptake at the injection site and inguinal and iliac lymph nodes. There was no abnormal accumulation of tracer in thorax or abdomen.

Duodenal biopsy (10X) showing dilated lymphatics visualised in the submucosal layer with preserved villous architecture and absent inflammation. (black arrow indicates dilated lymphatics).
In hospital treatment included therapeutic paracentesis of pleural fluid (500 ml fluid removal thrice). A low fat diet with Medium Chain Triglycerides (MCT) was started. A single dose of 600000 IU of intramuscular vitamin D was given with oral calcium 1000 mg/day. Thyroxine supplementation was given at a dose of 50 mcg/day. After three months of dietary measures, patient noted significant relief from abdominal distension and oedema.
Discussion
PIL is a rare disorder of the lymphatic system, described first as a series of 18 cases by Waldmann TA et al., [1]. It can present with anasarca, chronic diarrhoea, hypoproteinaemia, lymphoedema and chylous effusions [2]. Tuberculosis, filariasis, chest trauma, malignancies and haematological disorders usually contribute to most cases of secondary lymphangiectasia and chylous effusions and their absence is must for diagnosis of this condition [3]. Previously described as a paediatric disease, a variable age at diagnosis was later noted. Sporadic disease was found to account in 90% of cases. Familial basis was noted in the remaining 10% of the cases [4]. Intestinal lymphangiectasia is found to be associated with various genetic and acquired diseases [2,4,5]. We could not find evidence of these diseases in our patient.
Currently, postulated mechanism of lymphoedema and protein loss involves pressure abnormalities in lymphatic channels due to congenital anomaly of development or obstruction due to secondary causes. An increase in intraluminal pressure in lymphatics leads to dilatation in the intestinal wall which leads to leakage of protein and consequent hypoproteinaemia [5]. The protein-losing enteropathy accounts for anasarca, lymphopenia and immunoglobulin deficiency. Lymphoedema and chylous effusions also result from chronic lymphatic leakage [5].
Patients with PIL usually present in the first decade of life with symptoms resulting from chronic protein loss and lymphatic leakage. Oedema, diarrhoea, malabsorption and poor weight gain are features most commonly documented in previous reports [2,4]. Our patient had malabsorption but no diarrhoea. Pulmonary lymphangiectasia may coexist with intestinal disease, resulting in chylothorax associated with chylous ascites [6].
Ultrasound in case of PIL may reveal bowel wall oedema, ascites, dilatation of bowel loops and mesenteric oedema [2]. Contrast CT imaging may show ascites, diffuse bowel wall thickening and oedema due to dilated lymphatics. Lymphoscintigraphy may show absent or poorly developed lymphatics [7]. Confirmation of PIL requires histopathological examination. Endoscopy reveals yellowish dilated villi coating the intestinal surface. This finding is often missed on routine endoscopy in localised PIL and requires capsule endoscopy [8]. Biopsy findings include dilated lymphatics in any of the layers of the bowel wall. Normal plasma cells, minimal inflammation and mild villous atrophy are findings previously described [9]. Exclusion of secondary causes is vital to PIL diagnosis.
Treatment of PIL ranges from simple dietary measures to abdominal surgery. A low fat diet reduces absorption and pressure in lymphatics. MCT is directly absorbed into the portal circulation and is absorbed effectively in patients with PIL. Response to low fat, MCT diet is variable [10]. Dramatic improvement occurs in certain cases, while others require additional therapy or surgical treatment. Variable response to MCT rich diet results from its poor action on already dilated and damaged intestinal lymphatics. Periodic albumin infusions and therapeutic paracentesis may be required for treatment of ascites and pleural effusions.
Conclusion
The combination of malabsorption, anasarca and chylous effusions is a strong pointer towards intestinal lymphangiectasia. Diagnosis requires high degree of suspicion and endoscopic biopsies are sufficient for confirmation. Exclusion of secondary causes of intestinal lymphangiectasia is paramount to the diagnosis of PIL, especially in areas with a high prevalence of tuberculosis and filariasis.
[1]. Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS, The role of the gastrointestinal system in “idiopathic hypoproteinemia.”Gastroenterology 1961 41:197-207. [Google Scholar]
[2]. Vignes S, Bellanger J, Primary intestinal lymphangiectasia (Waldmann’s disease)Orphanet J Rare Dis 2008 3:5 [Google Scholar]
[3]. McGrath EE, Blades Z, Anderson PB, Chylothorax: aetiology, diagnosis and therapeutic optionsRespir Med 2010 104(1):01-08. [Google Scholar]
[4]. Alshikho MJ, Talas JM, Noureldine SI, Zazou S, Addas A, Kurabi H, Intestinal lymphangiectasia: Insights on management and literature reviewAm J Case Rep 2016 17:512-22. [Google Scholar]
[5]. Ingle SB, Hinge Ingle CR, Primary intestinal lymphangiectasia: MinireviewWorld J Clin Cases 2014 2(10):528-33. [Google Scholar]
[6]. White JE, Veale D, Fishwick D, Mitchell L, Corris PA, Generalised lymphangiectasia: pulmonary presentation in an adultThorax 1996 51(7):767-68. [Google Scholar]
[7]. So Y, Chung JK, Seo JK, Ko JS, Kim JY, Lee DS, Different patterns of lymphoscintigraphic findings in patients with intestinal lymphangiectasiaNucl Med Commun 2001 22(11):1249-54. [Google Scholar]
[8]. Chamouard P, Nehme-Schuster H, Simler JM, Finck G, Baumann R, Pasquali JL, Videocapsule endoscopy is useful for the diagnosis of intestinal lymphangiectasiaDig Liver Dis 2006 38:699-703. [Google Scholar]
[9]. Abramowsky C, Hupertz V, Kilbridge P, Czinn S, Intestinal lymphangiectasia in children: a study of upper gastrointestinal endoscopic biopsiesPediatr Pathol 1989 9(3):289-97. [Google Scholar]
[10]. Tift WL, Lloyd JK, Intestinal lymphangiectasia long term results with MCT dietArch Dis Child 1975 50:269-76. [Google Scholar]