During the past decade, surfactant administration has become the standard of care for treatment of premature neonates with severe Respiratory Distress Syndrome (RDS). In this group of patients, Randomized Controlled Trials (RCTs) and meta-analyses clearly demonstrate improved gas exchange following surfactant instillation, as well as significantly reduced neonatal morbidity and mortality [1,2].
Surfactant function is also inhibited by proteins and reactive oxygen metabolites released by leucocytes and bacteria [3-6]. In recent clinical trials, improved gas exchange following surfactant treatment was demonstrated in both term and preterm neonates with respiratory conditions other than surfactant deficient RDS, such as meconium aspiration syndrome, bacterial pneumonia and bronchopulmonary dysplasia [7-12].
Congenital pneumonia is a significant cause of morbidity and mortality in early neonatal period [13,14]. In a rabbit model of immature newborn rabbits with experimental Group B Streptococcal (GBS) pneumonia, Herting E et al., demonstrated that surfactant replacement improved lung function and mitigated bacterial growth [15]. In a nonrandomized controlled trial of preterm and term infants with respiratory failure and GBS infection, surfactant therapy improved gas exchange in a majority of patients with GBS pneumonia [8].
However, at present, there is no evidence from Randomized Controlled Trials (RCTs) to support or refute the efficacy of surfactant in term and late preterm infants with proven bacterial pneumonia. The lack of high-level evidence precludes any recommendation on the use of surfactant for bacterial pneumonia in term and late preterm infants [16].
This study was designed to evaluate whether surfactant therapy improves oxygenation and reduces short term pulmonary morbidity in late preterm and term neonates who are ventilated for Early Onset Pneumonia (EOP).
Materials and Methods
This prospective interventional cohort study was conducted at the tertiary care neonatal unit of Bharati Vidyapeeth University Medical College and Hospital, Pune, India, from January 2015 to December 2015.
Late preterm (gestational age 34+0- 36+6 weeks) and term (37+0-41+6 weeks) neonates with EOP requiring mechanical ventilation for respiratory failure, were included in the study. Neonates with major congenital malformations and respiratory diagnoses other than pneumonia were excluded. The study was approved by the Institutional Ethical Committee and written informed consent was obtained from the parents.
The diagnosis of pneumonia was based on guidelines recommended by the National Neonatology Forum of India [17]. A similar definition was also used in a prospective study by Mathur N et al., [14] and a review article on neonatal pneumonia [18].
EOP was defined as respiratory distress appearing within 72 hours of birth [18-20], with radiological features of pneumonia as reported by a blinded radiologist, with at least one risk factor for infection (maternal fever >38ºC / prolonged rupture of membranes >18 hours/maternal UTI/ suspected or confirmed chorioamnionitis/ spontaneous preterm labour) or neonatal blood culture positive for a proven pathogen.
Radiological findings were interpreted as per criteria described by Haney PJ et al., from chest films of 30 infants with autopsy-proven pulmonary infections (bilateral dense alveolar densities with air bronchograms, patchy segmental densities, granular densities, pleural effusions) [21].
Sepsis screen was considered positive if any two of the following laboratory parameters were abnormal–Total Leukocyte Count (TLC) <5000/mm3, Absolute Neutrophil Count (ANC) <1800/mm3, immature/total neutrophil (IT) ratio >0.2, platelet count <100,000/ mm3 or C reactive protein > 10 mg/L) [17].
Clinical pneumonia was defined as respiratory distress with radiological signs of pneumonia. Proven pneumonia was defined as clinical and radiological signs of pneumonia with positive sepsis screen and/or bacterial infection proven by culture.
In cases of pneumonia as defined above, the criteria for administration of surfactant included any one of the following: Fraction of Inspired Oxygen (FiO2) > 0.5 to achieve preductal SpO2 > 90%, Mean Airway Pressure (MAP) >8 cm H2O, arterial to alveolar PO2 ratio (a/A) < 0.25 or Oxygenation Index (OI) > 8.
Neonates meeting inclusion criteria received bolus doses of natural (bovine or porcine) surfactant at a dose of 100 mg/kg of phospholipids. Repeat doses were given if indicated after 6-12 hours. Surfactant was instilled into the trachea via an endotracheal tube using a feeding tube. Supportive management and antibiotics were given as per unit policy.
OI, a/A ratio, MAP and FiO2 were extracted from arterial blood gases obtained before and after surfactant therapy (1 and 12 hours post surfactant). Data regarding clinical outcomes such as duration of ventilation, oxygen therapy, survival rate, duration of hospital stay and complications were collected and analysed.
Statistical Analysis
Qualitative data were presented as frequencies and percentages, whereas quantitative data were presented as mean, standard deviation, or median and range. Wilcoxon signed rank sum test was used for assessment of change in oxygenation variables 12 hours after surfactant therapy. The data was analysed using SPSS version 20.0. The p-value of <0.05 was considered as statistically significant.
Results
During the 12 month study period, 1311 neonates were admitted to the unit. Eighty-two late preterm or term neonates were diagnosed with early onset pneumonia. Twenty four neonates were included in the study [Table/Fig-1].
Flow diagram for inclusion and exclusion.
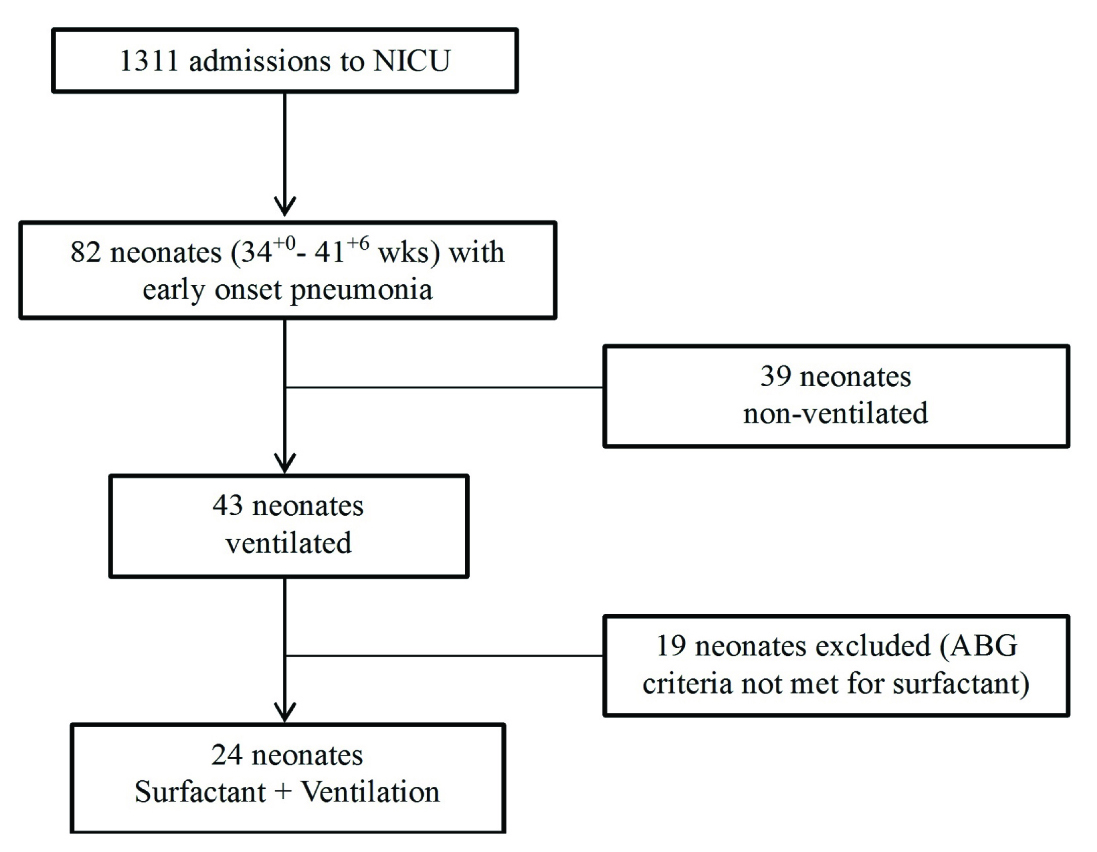
Demographic Characteristics
[Table/Fig-2] summarises the demographic characteristics of the 24 neonates.
| Characteristics | Value |
|---|
| Gestational age (wk) * | 36.5(±1.36) |
| Admission Weight (g) * | 2280(±370) |
| Male/Female (n) | 13/11 |
| Inborn/Outborn (n) | 11/13 |
| Term/Late preterm (n) | 13/11 |
| AGA/SGA (n) | 15/9 |
values are mean (± standard deviation)
wk-weeks, n-number, AGA-Appropriate for Gestational Age, SGA-Small for Gestational Age
Clinical and Intervention Characteristics
The median age of onset of respiratory distress was at birth i.e., 0 hours (range 0-20). The median age at admission was 19 hours (range-0-70) [Table/Fig-3]. The median age at intubation and surfactant administration was 26 and 34 hours respectively. One neonate received a second dose of surfactant.
Clinical presentation and intervention data (n=24).
| Clinical pneumonia n (%) | 18 (75) |
| Proven pneumonia n (%) | 6 (25) |
| Age at admission (h) † | 19 (0-70) |
| Onset of respiratory distress (h) † | 0.00 (0-20) |
| Age at intubation (h) † | 26 (0-70) |
| Age at surfactant therapy (h) † | 34 (12-70) |
values are median (range) h-hours
Change in Oxygenation
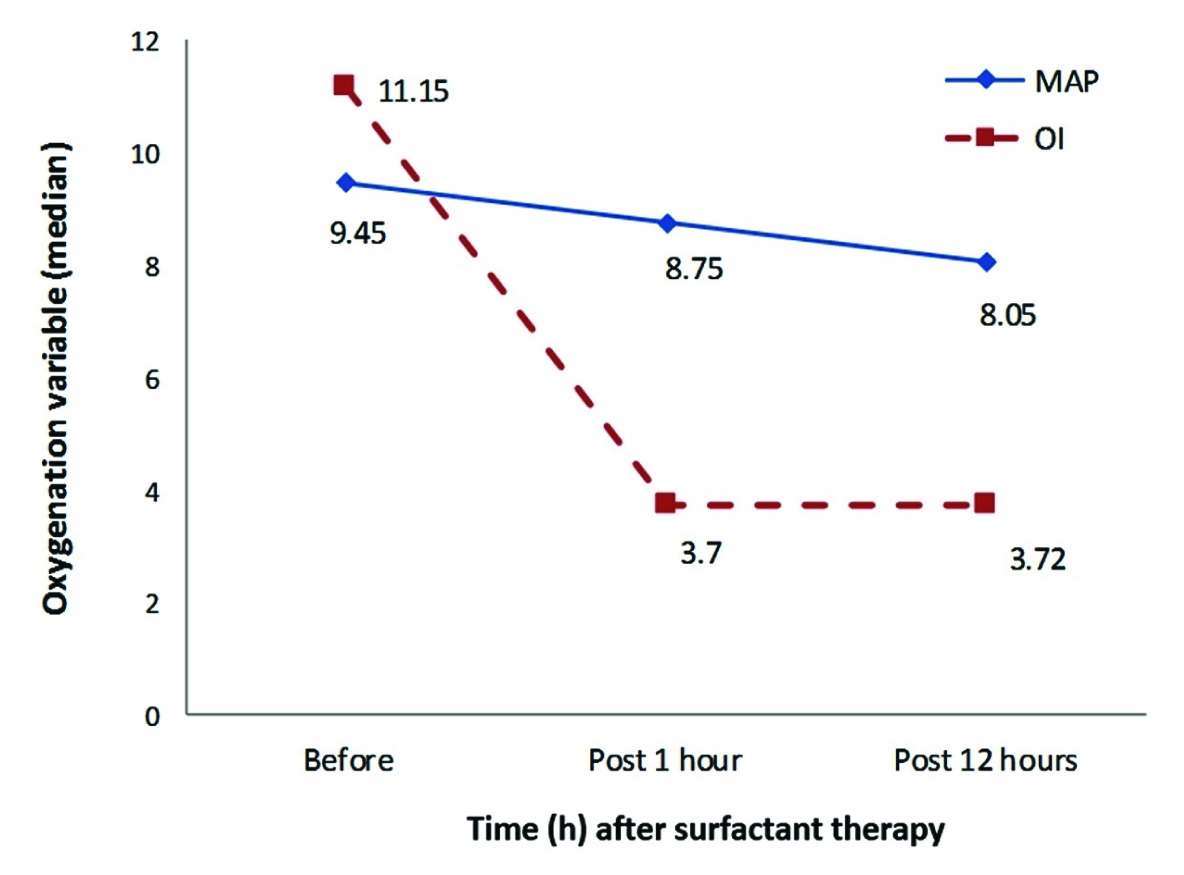
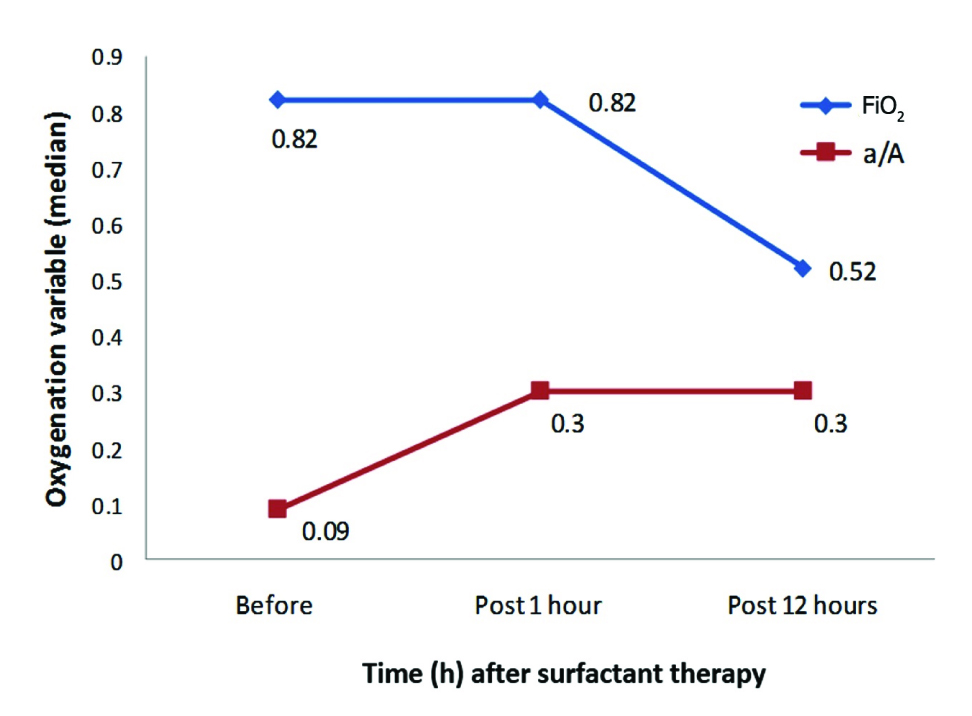
[Table/Fig-4,5] depict the change in oxygenation variables at 1 and 12 hours after surfactant administration. The p-value for the change in these variables before and 12 hours after surfactant therapy are given in [Table/Fig-6]. The median Oxygenation Index (OI) decreased from 11.2 to 3.7 at one hour and the change was significant and sustained at 12 hours (p<0.05). The median FiO2 could not be reduced within one hour, but by 12 hours it could be reduced from 0.82 to 0.52 (p<0.01). The median arterial/alveolar PO2 ratio improved from 0.09 to 0.3 within 1 hour and the improvement was significant at 12 hours (p<0.01).
Change In Mean Airway Pressure (MAP) and Oxygenation Index (OI) post surfactant at 1 and 12 hours.
Change in Fraction of Inspired Oxygen (FiO2) and arterial/alveolar PO2 (a/A ratio) post surfactant at 1 and 12 hours.
Change in oxygenation variables post surfactant at 12 hours.
| Variables median (range) | Before surfactant | Post surfactant 12 hours | p-value ‡ |
|---|
| MAP (cm H2O) | 9.45 (7.1-24) | 8.05 (5-18) | < 0.05 |
| OI | 11.15(1.3-52.7) | 3.72 (1.4-33.0) | < 0.05 |
| FiO2 | 0.82 (0.25-1.00) | 0.52 (0.21-1.00) | < 0.01 |
| a/A ratio | 0.09(0.04-0.85) | 0.3 (0.05-1.76) | < 0.01 |
Wilcoxon signed rank sum test
MAP-Mean Airway Pressure, OI-Oxygenation Index, FiO2-Fraction of inspired Oxygen, a/A -Arterial /Alveolar
Outcome Following Surfactant Therapy
The study infants could be extubated after a median duration of 29 hours. Continuous Positive Airway Pressure (CPAP) support was given for a median duration of 82 hours and supplemental oxygen for 23 hours. None of the patients had air leaks or chronic lung disease. Two patients (8.3%) had grade I/II Intra-Ventricular Haemorrhage (IVH) and none had severe (grade III or IV) IVH. Median duration of hospital stay was 15 days. Survival at 28 days of age was 92% (22 neonates).
Discussion
In this prospective study of late preterm and term neonates with early onset pneumonia, we observed rapid improvement in oxygenation after surfactant replacement which was significant and sustained for a longer period.
Nearly half (54.2%) of neonates in our study were term, indicating that infants with mature surfactant levels in their lungs can also develop respiratory failure due to pneumonia soon after birth. In a study by Lotze A et al., on term infants with respiratory failure and at risk of requiring Extra-Corporeal membrane oxygenation (ECMO), a subgroup of infants with sepsis and respiratory failure showed improved oxygenation and reduced need for ECMO after surfactant [22]. Most of the other studies that have studied effectiveness of surfactant in sepsis or pneumonia included only preterm [23] or preterm as well as term infants [8].
Majority of neonates with early onset pneumonia in our study developed respiratory distress at birth, indicating that most often the infection is acquired in utero or in the intrapartum period. However, as 54.2% were outborn, the median age of admission was 19 hours. Only 25% had proven pneumonia, which indicates that a large number of neonates with pneumonia do not have a positive sepsis screen or blood culture. The median age at surfactant administration was 34 hours. Surfactant administration is not yet a standard of care in neonates with pneumonia. Along with cost consideration, this could be a factor leading to delay in using surfactant for pneumonia. In our study, only one infant received a repeat dose of surfactant. In a study by Vento G et al., in preterm infants with birth weight less than 1250 g, a significantly higher number of infants who had RDS with pneumonia, needed a second dose of surfactant, as compared to those with RDS alone [23]. The characteristics of population in their study (preterm, very low birth weight, with RDS and pneumonia), could explain the requirement of repeat doses of surfactant.
In our study, the median OI and arterial to alveolar PO2 (a/A) ratio showed a rapid improvement within one hour after surfactant treatment, which was sustained for a longer period and significant at 12 hours. This change was predominantly due to an improvement in median partial PaO2. No other prospective study has used a/A ratio or OI as a parameter to study improvement following surfactant therapy in neonates with pneumonia. In a prospective observational study, Herting E et al., studied the effects of surfactant treatment in preterm and term neonates with GBS pneumonia with respiratory failure and compared this with a control group of non-infected infants with RDS [8]. The GBS group showed improvement in gas exchange, although the response was slower than in infants with RDS. The median FiO2 in Herting’s study could be reduced from 0.84 to 0.5 in one hour. In our study, the FiO2 showed a relatively slower decline, most of it after one hour of surfactant administration. This slower reduction of FiO2 despite rapid improvement in oxygenation could be related to the study population. In Herting’s study, the majority of infants were very preterm or extremely preterm with a median gestational age of 30 (27-33) weeks; whereas our study patients included only term and late preterm infants. The fall in median MAP requirement was also gradual in our study which was similar to the findings in Herting’s study (MAP decreased from 13 cm to 12 cm H2O at 1 hour and 11.7 cm H2O at 12 hours following surfactant). Higher oxygen saturation targets in our study population of term and late preterm infants may have led to a more gradual weaning of FiO2 and ventilatory support. Also, initial MAP requirement was lower in our study, which may explain the relatively marginal decline.
The treatment was well tolerated as well. The longer duration of ventilation and higher rate of complications and mortality in the study by Herting E et al., could be related to inclusion of preterm infants with lower gestational ages (median gestational age 30 weeks) and GBS septicaemia [8]. As ours was not a randomized study, we can only speculate that by improving ventilation parameters rapidly, surfactant therapy may shorten the duration of ventilation and therefore reduce the cost of therapy and risk of complications of ventilation.
Pathophysiology of pneumonia involves leak of plasma proteins into the airways and accumulation of cytokines within the lung which exacerbates inflammatory response. Several studies have demonstrated that this proteinaceous material inhibits surfactant function [24-26]. Free radical production by bacteria may also cause lipid peroxidation of lung surfactant [5]. Facco M et al., studied endogenous surfactant kinetics of late preterm and term newborns with pneumonia. Half-life and pool size of the major component of surfactant, Disaturated-Phosphatidylcholine (DSPC) were markedly impaired in newborns with pneumonia [6]. This could explain the rapid improvement seen with exogenous surfactant administration. In vitro studies have demonstrated that addition of surfactant associated proteins to natural surfactant extracts makes the surfactant mixture more resistant to the inhibitory effects of plasma proteins such as fibrinogen, albumin and haemoglobin [25,26]. Future research could therefore include surfactant replacement products which contain surfactant peptides like SP-A, SP-B and SP-C derived from natural extracts or recombinant apoproteins, for treatment of pneumonia.
The strength of our study lies in the fact that this is the only prospective study on effects of surfactant treatment in early onset pneumonia, in a population of late preterm and term neonates. In addition, there are very few studies that have measured and analysed multiple indices of gas exchange such as OI and a/A ratio to assess the change in oxygenation after surfactant therapy for pneumonia. The study is not without its limitations though. The sample size of the study was rather small. Also, there was no control group to compare the effects of surfactant in pneumonia. Future large multi-centre randomized trials are needed to definitively address the risks and benefits of surfactant therapy in this group of neonates.
Conclusion
We conclude that significant improvement in oxygenation was seen after surfactant replacement therapy in late preterm and term neonates with early onset pneumonia. There may be a substantial role for this treatment in early onset neonatal pneumonia. However, in absence of controls, it cannot be stated with certainty that these changes are due to surfactant therapy and not the result of natural recovery due to supportive treatment. Larger randomized controlled trials are therefore needed before definite recommendations can be made.