Pyrexia of Unknown Origin (PUO) poses a great challenge for both diagnosis and clinical management [1]. The aetiological agents of this condition can vary, not only with the geographic region but also the seasons. In an earlier study from India, infectious diseases were found to be the most common cause of PUO, followed by neoplasms and collagen vascular diseases [2]. Studies from other countries indicated that 1.8%-19.3% of PUO’s due to infectious agents are caused by scrub typhus, an acute febrile illness caused by a Rickettsia Orientia tsutsugamushi [3]. Manifestations of scrub typhus are often confused with several other infectious PUO’s such as typhoid, leptospirosis, malaria, and dengue. It is important to differentiate these infections as their management differs [4]. Weil-Felix test, in combination with clinical diagnosis, has been in use since a long time for diagnosing scrub typhus, but the test suffers from poor sensitivity and specificity. Indirect Fluorescent Antibody (IFA) and indirect immunoperoxidase assays are considered the gold standards to diagnose the infection [5]. However, these tests are expensive and not readily available in India. Alternative methods like the ELISA specific for detecting Immunoglobulin M (IgM) antibodies and more recently lateral flow detection tests are being used to diagnose the condition [6]. These tests are reported to have varying levels of sensitivity and specificity, often leading to misdiagnosis. Polymerase Chain Reaction (PCR) is being increasingly used, as a more reliable and specific marker of the infection. Real-Time and nested PCR assays have been developed to detect various genes of O. tsutsugamushi, which include those targeting 56 kDa, 16S rRNA, 47 kDa, and the groEL genes, each having its own advantage and disadvantage [7]. Since, scrub typhus is endemic in many resource-poor developing countries, conventional PCR, targeting groEL is a suitable candidate for the application of molecular methods in settings where the cost of establishing a RT thermocycler is prohibitively high [8]. A conventional PCR, which is sensitive and specific, would be convenient, as it is cheaper and comparatively easy to perform. Hence, we developed a groEL gene-based conventional PCR assay after identifying a conserved fragment of the gene. This gene is widely distributed in the genome of both prokaryotes and eukaryotes and is a housekeeping gene, coding for groEL proteins, which are essential for the survival of these cells. Although the groEL gene is highly conserved, it is sufficiently variable to design molecular assays for differentiating the genera Orientia and Rickettsia [8]. Recently, the groEL gene has been used to identify Streptococcus, Enterococcus, Staphylococcus, Bartonella, Mycobacterium, and Ehrlichia, and also for deriving evolutionary relationships among the members of the eubacterial lineage [8-14]. Therefore, in this study, we developed a groEL gene-based conventional PCR assay after identifying a conserved fragment. Further, we compared the detection rates of three molecular markers of O. tsutsugamushi, namely 56 kDa, 16S rRNA, and groEL genes by PCR, along with IgM ELISA from blood samples of patients and attempted to identify a single test or a combination which could give enhanced sensitivity and specificity.
Materials and Methods
This is a prospective study that was conducted at Vector Control Research Centre, Puducherry, India. Samples were collected from patients coming to Pondicherry Institute of Medical Sciences (PIMS), Puducherry, between March 2011 and March 2013, and the study was approved by the Institutional Ethical Committee of PIMS (No. IEC/RC/12/54). One hundred and forty-five consecutive cases with fever, who reported to the hospital, were included in the study.
Inclusion criteria were fever for more than five days, with an eschar, rash or lymphadenopathy or fever for which the cause was not known. Cases with fever, for which the cause was already known and had no eschar were excluded from the study. Duration of fever and the presence of eschar in each patient were noted down. Venous blood was collected from these patients after obtaining written informed consent and serum separated was used for IgM ELISA, while blood clots were used for DNA extraction. PCR was performed on 145 samples to detect the presence of three genes of O. tsutsugamushi mentioned above.
Detection of IgM Antibodies
This test was used to screen samples to detect the infection. Scrub typhus detect kit, which utilizes a recombinant antigen of Orientia to detect IgM antibodies by ELISA (InBios International Inc., Seattle, USA) was used for this purpose. The dilution of test sample used was 100 µL of 1:100 diluted serum. The cut off was 0.406, which was calculated based on Optical Density (OD) values from 300 samples of known scrub typhus positives, normal sera and sera from other fevers. A Reciever Operating Curve (ROC) was drawn to arrive at the cut off (data submitted for publication). Samples with OD value more than the cut off were considered positive for O. tsutsugamushi specific IgM antibodies. used as template in the second round of PCR in the same volume of reaction with a second set of forward and reverse primers. The cycling conditions were similar for the first and the second round of PCR, and the cycles were 95°C for 10 seconds, 57°C for 30 seconds, and 72°C for 1 minute, which was repeated 30 times, in a Mastercycler Personal Thermocycler (Eppendorf, Germany).
Detection of Gene Encoding 16S rRNA
The primers used to detect 16S rRNA were described by Sonthayanon W et al., [Table/Fig-1] [16]. The reaction mixture contained Green Master Mix (Promega, USA) with 10 pmol each of forward and reverse primers and 4 µL of extracted DNA in a final volume of 30 µL. The cycling conditions were 95°C for 1 minute, 61°C for 1 minute, and 72°C for 30 seconds repeated for 35 cycles, in a Master Cycler Gradient Thermocycler (Eppendorf, Germany). The presence of amplicons from all three PCRs was visualized in a gel documentation system (Gel Doc IT Imaging System, UVP) after electrophoresis in 1.5% agarose gel containing ethidium bromide (0.5 g/mL).
Details of primers used for the detection of three genes of Orientia by PCR.
| Gene detected | | Details of primer | Product size |
|---|
| groEL (designed in primer 3) | Forward | 5’-TTG CTG ATG ATG TAG ACG GA-3’ | 300 bp |
| Reverse | 5’-TGT TCA CAA CGA GAA TTA ACT T-3’ |
| 56 kDa [15] nested PCR | (Cycle 1) ForwardReverse | 5’-TCA AGC TTA TTG CTA GTG CAA TGT CTG C -3’5’-AGG GAT CCC TGC TGC TGT GCT TGC TGC G -3’ | 583 bp |
| (Cycle 2) ForwardReverse | 5’- GAT CAA GCT TCC TCA GCC TAC TAT AAT GCC - 3’5’- CTA GGG ATC CCG ACA GAT GCA CTA TTA GGC - 3’ |
| 16S rRNA [16] | Forward | 5’- CGA ATT AAT GCT GAG TTT GCT TAG - 3’ | 220 bp |
| Reverse | 5’-CTC TCA GAC CAG CTA CAG ATC ACA - 3’ |
PCR: Polymerase Chain Reaction; bp: base pairs.
Nucleic Acid Sequencing
The PCR products were confirmed to be that of Orientia based on nucleic acid sequencing. Amplicons from 56 kDa, 16S rRNA, and groEL PCR were sequenced using the respective forward or reverse primers and Big Dye terminator (Applied Biosystems, CA, USA). Sequences were obtained in an ABI automated genetic analyzer 3130 XL (ABI – Applied Biosystems, CA, USA), edited using Bio Edit (Version 7.0.0), Basic Local Alignment Search Tool (BLAST) analyzed in the NCBI website and confirmed as the respective genes of O. tsutsugamushi.
The results of PCR assays were analyzed and the association between the duration of fever and the detection rates of the diagnostic tests was tabulated. Furthermore, an algorithm to find out the best test or best possible combination of tests for detection of the disease was also made.
DNA Extraction
DNA from blood clot was extracted using the Gen Elute blood genomic extraction kit (Sigma – Aldrich) and as per the protocol provided in the kit and stored at -20°C until further use.
Detection of Gene Encoding groEL
The primers used in this PCR were designed based on conserved region of groEL gene, using Primer 3 software. The conserved region of the gene (NT 200-520) was identified by aligning 40 nucleotide sequences of groEL gene of O. tsutsugamushi available in the National Centre for Biotechnology Information (NCBI) database, and these sequences belonged to isolates from endemic regions such as China, Japan, Vietnam, Korea, and Thailand. The gene fragment showed 100% similarity between the sequences included in the analysis. The details of primers used are given in [Table/Fig-1].
The PCR reaction mixture contained Green Master Mix (Promega, USA), 10 pmol each of forward and reverse primers and 4 µL (50 ng/mL) of extracted DNA in a final volume of 30 µL. The cycling conditions were 30 cycles of 95°C for 20 seconds, 52°C for 45 seconds, and 72°C for 1 minute in a Master Cycler Gradient (Eppendorf, Germany).
Detection of Gene Encoding 56 kDa
Nested PCR was performed using the method and primers described by Saisongkorh W et al. [15] [Table/Fig-1] with minor modification in the protocol. The PCR amplification mixture contained Green Master Mix (Promega, Madison, USA) with 10 pmol each of forward and reverse primers and 1 µL of extracted DNA in a final volume of 25 µL. One microliter of PCR product was
Statistical Analysis
The data obtained were entered into the Microsoft Excel spreadsheet and exported to IBM-Statistical Packages for Social Sciences software version 16.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis was performed using Chi-square test and Fisher’s exact test. The level of significance was set at p<0.05.
Results
One hundred and forty-five suspected cases of scrub typhus were included in the study, out of which 135 had IgM antibodies to O. tsutsugamushi and the remaining 10 although negative for IgM antibodies, had high-grade fever with the characteristic eschar.
Other common causes of febrile illnesses that occur in this region such as malaria, typhoid, and dengue were ruled out based on appropriate laboratory investigations as follows; malaria was ruled out based on microscopic examination of stained peripheral blood smear and parascreen rapid test for malaria (Zephyr Biomedicals, India). Dengue was ruled out based on dengue day 1 test (J. Mitra, India) and typhoid by both conventional blood culture and Widal test using stained Salmonella antigens (Span, India).
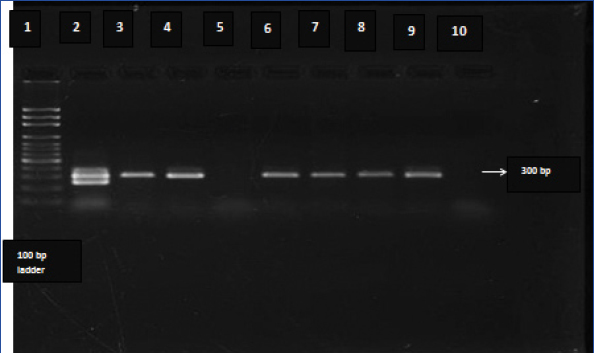
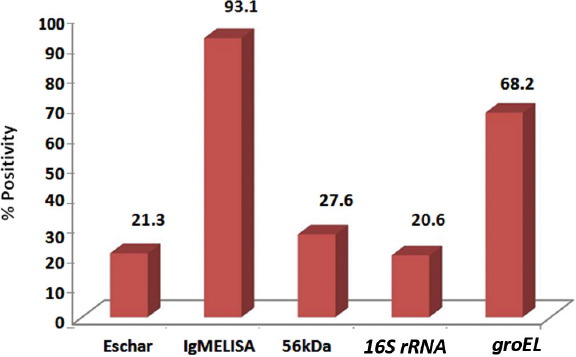
All 145 samples were further subjected to PCR to detect three genes of O. tsutsugamushi, viz., 56 kDa, 16S rRNA, and groEL. The results of PCR showed that 40, 30, and 99 samples were positive, respectively, by 56 kDa, 16S rRNA, and groEL gene-based PCR and the positivity of the respective assays worked out to 27.6%, 20.6%, and 68.2%. The gel electrophoresis picture for detection of groEL gene is shown in [Table/Fig-2]. The amplicons of the three assays were sequenced and confirmed by BLAST analysis. Eschar, a characteristic lesion associated with scrub typhus was seen only in 21.3% of the cases, while IgM ELISA was positive in 93.1% of the cases [Table/Fig-3].
Gel electrophoresis picture of amplicons obtained in groEL polymerase chain reaction (PCR). Lane 9 is the positive control and Lane 10 the negative control. Lanes 2, 3, 4, 6, 7 and 8 show amplification products in the PCR while Lane 5 shows sample with no amplification.
Percentage positivity of different diagnostic markers/assays of scrub typhus. The tests were performed on samples from suspected cases.
The specificity of the three PCR assays was tested on DNA from blood clots from confirmed cases of malaria, dengue, and typhoid. Three samples from each of the three diseases were subjected to 16S rRNA and 56 kDa PCR, and 10 samples each were tested by groEL PCR and all of them were negative.
Nucleic Acid Sequencing and Phylogenetic Analysis
Amplicons of each gene from the three PCR’s were sequenced for confirmation. Nucleotide sequences of amplicons of the three genes have been submitted to Gen Bank, and accession numbers obtained (KT 970942, 43; KU 365725 and KU 365726). To the best of our knowledge, this is the first report of groEL gene being detected from scrub typhus cases in India.
The sensitivity of 56 kDa PCR and 16S rRNA PCR was 27.6% and 20.6%, respectively, whereas the sensitivity of groEL PCR developed by us was 68.2%. An analysis to show the association between the duration of fever in the subjects and the detection rates of the diagnostic tests is shown in [Table/Fig-4].
Duration of fever versus positivity by various diagnostic markers/tests (n=145) showing that both IgM ELISA and groEL PCR could pick up positive samples both during the acute phase and beyond 10 days of illness.
| Days of fever | Total number of samples | Eschar | IgM ELISA | 16S rRNA PCR | 56 kDa PCR | groEL PCR |
|---|
| <7 | 48 | 9 | 43 | 12 | 16 | 36 |
| 7-10 | 58 | 13 | 56 | 10 | 12 | 36 |
| >10 | 39 | 9 | 36 | 8 | 12 | 27 |
IgM: Immunoglobulin M, ELISA: Enzyme-Linked Immunosorbent Assay, PCR: Polymerase Chain Reaction.
Out of the samples included in the study, 48 were collected, during the first week of fever out of which, 43 were positive by IgM ELISA (89.58%), 36 by groEL PCR (75.00%), 16 by 56 kDa nested PCR (33.33%), and only 12 by 16S rRNA PCR (25.00%). Fifty-eight samples were collected during days 7-10 of fever and 56 (96.55%), 36 (62.06%), 12 (20.68%), and 10 (17.24%) samples were positive by the respective diagnostic assays. However, among the molecular diagnostics, the positivity was highest by groEL PCR in samples collected beyond day 10. Thus, groEL PCR gave higher positivity than 56 kDa nested and 16S rRNA PCR and the positivity by the former increased significantly in samples collected beyond 10 days of onset of fever. On the other hand, the positivity with 56 kDa and 16S rRNA PCR decreased beyond one week. Most interestingly, all 10 IgM ELISA negative samples were positive by groEL PCR, while the 56 kDa nested and 16S rRNA PCRs detected some of them as negative.
Comparison of Performance of Combinations of Different Diagnostic Methods
To arrive at a combination of diagnostics that would give highest sensitivity for detecting the infection, we performed descriptive type of statistical analysis and checked the number of samples testing positive by different combinations of the five diagnostics, viz., Eschar, IgM ELISA, and 56kDa, 16S rRNA, groEL PCRs. The salient features of this analysis are shown in [Table/Fig-5].
Performance of significant combinations of diagnostics for scrub typhus indicating that IgM ELISA was positive in maximum number of samples followed by groEL PCR.
| Eschar | IgM | 56 kDa | 16S rRNA | groEL | Total positive* |
|---|
| + | | | | | 31 |
| - | | | | + | 99 |
| - | + | | | | 135 |
| + | | | | + | 24 |
| - | + | | | + | 89 |
| + | + | | | | 21 |
Total number of samples tested=145, ‘+’ indicates considered for comparison and ‘-’ indicates not considered for comparison.
IgM: Immunoglobulin M, ELISA: Enzyme-Linked Immunosorbent Assay, PCR: Polymerase Chain Reaction.
O. tsutsugamushi-specific IgM ELISA antibody test alone detected 135 samples (93.1%) as positive whereas groEL PCR alone tested 99 (68.2%) samples as positive. On the other hand, a combination of these two diagnostics tested 89 samples (61.3%) as positive. Thus, the analysis indicated that an algorithm that could pick up maximum number of positives is as follows; test suspected case of scrub typhus for IgM antibodies and if found positive confirm the diagnosis as scrub typhus. If IgM is negative perform groEL PCR and if positive decide the diagnosis as scrub typhus. If both IgM ELISA and groEL PCR are negative, but the patient has high-grade fever with an eschar then it can be construed that the case is scrub typhus and the tests may be repeated after a few days for a definitive diagnosis.
In this study, 142 cases responded to doxycycline treatment, and the remaining threes cases reported to the hospital in later stages of the disease, and were not on any antibiotics and succumbed to myocarditis, severe liver dysfunction and shock.
Discussion
There have been increasing reports of scrub typhus from India in the recent past, and serological tests form the mainstay of diagnosis of the infection [17-20]. Owing to the lack of availability of the gold standard tests in the country, the alternative, widely used test to detect Rickettsial antibodies, the Weil-Felix test is still being used in the diagnosis of these infections. This test is reported to have high specificity but low sensitivity, at a breakpoint of 1:80, the test offers a specificity of 100% but a sensitivity of only 30% [19,20]. A study from Southern India reported the test to be 94% specific and 59% sensitive and is said to be highly suggestive when interpreted with proper clinical correlation [21]. Another diagnostic assay being used is the ELISA to detect IgM antibodies, the sensitivity and specificity of which is reported to vary according to the geographic area [18,19,21]. This test is reported to provide positive results within 3-4 days of onset of illness, but problems with this are the availability and the cost of the kit [19].
Alternatively, molecular methods have been used for diagnosis, and detection of 56 kDa gene has been widely reported. The sensitivity for detection of this gene is reported to be 29% and specificity about 99%. The reported detection limit is approximately 100 fg of DNA. The lower sensitivity of this PCR reported from earlier studies, and relatively lesser detection rates in the present study are probably because of the genetic variability associated with the gene and hence the inability to detect all the cases. Detection of 16S rRNA is reported to be about 45% sensitive and 99.7% specific [3,15]. However, in this study, least number of samples was detected as positive by this PCR. Although the gene coding for 16S rRNA is said to be conserved among different strains, the reason for the low positivity as compared to the other two PCR’s could be because of the bacterial copy number, which varies within a wide range, from 1000 to 29,000/L of blood [3] and the bacterial load in the negative samples could be low and hence not detectable.
The groEL gene is highly conserved and encodes a heat shock chaperonin present in bacteria [22]. This gene has been targeted in the diagnosis of rickettsial infections by real time and duplex-PCR in earlier studies [8,23]. In this study, this gene was detected using conventional PCR and positivity of the cases was maximum by this method compared to the other gene-based diagnostics. The higher level of detection of groEL gene in this study could be due to the gene, being highly conserved among Orientia; the nucleotide identity among them ranging between 95.4% and 99.1% [23]. Further, in this study, groEL PCR was done on DNA from samples known to be positive for other common fevers such as dengue, malaria, and typhoid and also with DNA extracted from normal individuals and none of these samples tested positive, indicating its high specificity. The expression of this gene is known to indicate stress at the cellular level and is upregulated in the acute phase of the infection in the bacterial genome [8,22]. In comparison to the other two methods, groEL PCR could identify the presence of the bacterium throughout the course of the infection [Table/Fig-4]. Statistical analysis using Chi-square and Fisher’s exact test showed that the following tests were significant: IgM ELISA significantly tested more number of positives in comparison to the eschar or the three PCR’s (p≤0.001). The groEL PCR significantly tested more number of positives than the number of cases with an eschar and also the other two PCR’s (p≤0.001). However, when the performance of different diagnostic markers, i.e., the eschar and the diagnostic assays, was considered, the positivity of cases with an eschar alone was 21.7%. Cases with both eschar and groEL PCR positivity was 29.03%, indicating that about one-third cases with eschar, tested positive by this assay. Similarly, the number of cases, positive for both eschar and IgM antibodies was also 29.03%, while cases, which had eschar together with positive IgM and groEL PCR was only 9.6%. Taking into account cases that were IgM and groEL PCR positive, which was 61.4%, and this indicates that a combination of these two assays could definitely pick up more number of cases with high specificity. The presence of eschar further enhances the sensitivity, as also specificity. Positivity of groEL PCR alone was 68.3% while that of IgM ELISA was 93.1%. This difference in positivity between the two tests could be due to antibiotic treatment. All the patients who had IgM antibodies and negative groEL PCR had been administered antibiotics leading to the clearance of the organisms from blood. Considering the inherent limitations of different diagnostics of scrub typhus, which are due to the presence or absence of respective markers (eschar, antibodies or DNA), which appear during different stages of the disease, the choice of the diagnostic is tricky. This is also evidenced in the present study. The presence of eschar is considered pathognomonic of scrub typhus [20]. However, eschar is a nonspecific marker as it can occur in gangrene, ulcers, fungal infections, necrotizing spider bite wounds, spotted fevers, and cutaneous anthrax. Further, eschar has been reported in as few as 7% to as high as 97% of scrub typhus cases, and cutaneous manifestations of scrub typhus may not always be the classical eschar [24-26]. Similarly, the 47 kDa, 56 kDa, and 16S rRNA-based PCR assays also do not have adequate sensitivity [7,15,16]. IgM ELISA which uses recombinant 56 kDa antigen is reported to have varied sensitivity. Blacksell SD et al., obtained a sensitivity of 93% and specificity of 91% (Inbios) with a cut off of 0.5 OD, when improved and more-stringent scrub typhus infection criteria were applied and after correcting for low false-positive IFA titers. This study suggested that regional cut off should be worked out for broader application [27]. Prakash JA et al., have reported a sensitivity of 86% for IgM ELISA (Pan Bio) and observed cross reactivity with falciparum malaria, pulmonary tuberculosis, Streptococcus viridans septicemia, and typhoid fever [28]. Another study observed that the test had 90% sensitivity and specificity and provided positive results within 3-4 days of the onset of illness [18]. Gupta N et al., reported a sensitivity and specificity of 100% and 94.12%, respectively, at a ROC based cut off of 0.87 OD [29]. Recently, published guidelines of Indian Council of Medical Research recommend a cut off value of 0.5 [30]. Hence, a uniform cut off value for different regions in India appears not applicable, while working out a cut off for each region/area may not be feasible. In view of this, the results of ELISA based assays remains questionable. Added to this, even if we consider 94% sensitivity and 91% specificity, the ELISA leaves a margin for false negatives/positives, although small but critical for the treatment of cases. Hence, conventional groEL PCR seems to be an ideal alternative particularly in the early stage of the disease and in cases that have not been started on antibiotics.
Limitation
The main limitation of this study was that patients on antibiotics were also included, which would bring down the sensitivity of PCR. In the present scenario, where antibiotics are prescribed for fevers without any delay, the most appropriate combination of tests would be that of conventional groEL PCR together with IgM ELISA, which would enhance the sensitivity and specificity, thus aiding in accurate diagnosis of scrub typhus.
Conclusion
This study suggests that IgM ELISA could be used to diagnose the infection particularly after five days of onset of fever, while detection of groEL gene by conventional PCR could be used for diagnosis within five days of fever and for confirmation in case of untreated cases throughout the course of the infection.
Financial Support
The authors acknowledge the financial support extended by Indian Council of Medical Research in the form of intra mural research funding for this study.