Study of Red Cell Fragility in Different Stages of Chronic Kidney Disease in Relation to Parathyroid Hormone
Suchismita Panda1, Anuva Mishra2, Manoranjan Jena3, Sashi Bhusan Rout4, Srikrushna Mohapatra5
1 Associate Professsor, Department of Biochemistry, PRM Medical College, Baripada, Odisha, India.
2 Associate Professsor, Department of Biochemistry, VSS Medical College, Burla, Odisha, India.
3 Assistant Professor, Department of Social and Preventive Medicine, SCB Medical College, Cuttack, Odisha, India.
4 Professor, Department of Nephrology, SCB Medical College, Cuttack, Odisha, India.
5 Professor, Department of Biochemistry, PRM Medical College, Baripada, Odisha, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Suchismita Panda, N3/206, IRC Village, Nayapalli, Bhubaneswar-751015, Odisha, India.
E-mail: dr.suchismitapanda17@gmail.com
Introduction
Anaemia is one of the common complications associated with Chronic Kidney Disease (CKD) responsible for the increase in the morbidity and mortality in such patients. Several factors have been attributed to cause renal anaemia, amongst which hyperparathyroidism is one of the less recognised reasons. Most studies have been conducted in this regard in CKD patients undergoing haemodialysis. The level of PTH in early stages of chronic kidney disease has not been much studied. The excess amount of Parathyroid Hormone (PTH) secondary to CKD has been suggested to be a causative factor for anaemia.
Aim
To evaluate the serum PTH level in CKD patients before haemodialysis and to study the association of the haemoglobin status with the parathyroid hormone.
Materials and Methods
Forty CKD patients above 18 years of age before haemodialysis and 25 age and sex matched healthy controls were included in the study. Routine biochemical and haematological parameters such as Routine Blood Sugar (RBS), urea, creatinine, Na+, K+, Ca2+, PTH and Hb% were perfomed. Red cell osmotic fragility was measured by serial dilutions of whole blood with varying concentrations of sodium chloride ranging from 0.1% to 0.9%.
Results
The study revealed a significant fall in Hb%, along with a rise in Median Osmotic Fragility (MOF) and PTH in the CKD patients when compared to the control group. Linear regression of PTH with Hb% revealed significant negative association between both the parameters with a R2 value of 0.677. Multilinear regression analysis of MOF and other independent variables such as Hb%, Na+, K+, Ca2+, urea, PTH and creatinine highlighted the variance of MOF by 72%, maximal variance contributed by PTH. Receiver Operating Curve (ROC) analysis revealed an area under the curve of 0.980 with a sensitivity of 100% and specificity of 87% in detecting osmotic fragility at a cut off value of PTH ≥100 pg/ml.
Conclusion
The underlying cause of anaemia should be identified early in the CKD patients before haemodialysis. Secondary hyperparathyroidism should be ruled out as a causative factor of anaemia to slow down the progression of the disease process.
Anaemia, Hyperparathyroidism, Osmotic fragility
Introduction
Hyperparathyroidism is commonly associated with declining kidney function, due to alteration of the bone and mineral metabolism [1]. Altered levels of vitamin D and phosphate are responsible for this increase in PTH, which develops quite early in the course of the disease process and remains unrecognised and untreated. Anaemia is a common complication in CKD patients, who have a mortality rate 20-100 times higher than the normal population [2].
Several studies have revealed the association of hyperparathyroidism with CKD [3-5]. Parathyroid hormone (PTH) is a major uremic toxin which may be responsible for long term consequences in CKD such as renal osteodystrophy, vascular calcification, altered cardiovascular function, immune dysfunction and anaemia [6,7]. Hyperparathyroidism induces anaemia in patients with normal kidney function. Therefore, raised PTH secondary to chronic kidney disease may result in unfavourable influence on the haemoglobin level of uremic patients [8]. Patients undergoing haemodialysis have low haemoglobin which may be attributed to their PTH level as cited by various authors [9-11].
Secondary hyperparathyroidism is often an unrecognized incident and develops early in the course of the disease before dialysis initiation [12]. Correction of anaemia in the CKD patients is essential for the prevention of associated cardiac complications and other debilitating features such as fatigue, weakness, anaemia and sleep disturbance. Low haemoglobin levels also increases the length of the stay in the hospital leading to higher consumption of health care resources and increased risk of mortality [13,14]. Anaemia induced by PTH may be due to bone marrow fibrosis as suggested by Rao DS et al., [15] or suppression of peripheral burst forming units or by causing an enhanced osmotic fragility.
Though various pathophysiological mechanisms have been outlined by in vitro and in vivo studies, regarding the worsening effect of raised parathyroid levels on the haemoglobin levels, no definitive mechanism has been identified. The objective of the present study was to evaluate the serum PTH levels in various CKD patients before haemodialysis and assess the association of haemoglobin level with the hormone. The possible underlying mechanism is also studied by examining the fragility of the red blood cell at different concentrations of PTH.
Materials and Methods
The present study was undertaken in the Department of Biochemistry, in association with the Department of Nephrology of SCB Medical College, Cuttack, Odisha, India, during the period of August 2015 to August 2016. A total of 40 CKD patients attending the OPD or admitted to the indoors, above 18 years and 25 healthy age and sex matched controls who were healthy volunteers, were included in the study group.
Informed consent was obtained from cases and controls followed by medical history, clinical examination and routine biochemical investigations after Institutional Ethical Clearance. Those patients with stage 5 CKD requiring dialysis treatment and other associated kidney disease, therapy with iron, erythropoetin stimulating agents, associated bleeding disorders or need for blood transfusion which interfere with the haematocrit value, anaemia due to other causes such as Vitamin B12, folate, iron deficiency, haemoglobinopathies were excluded from the study.
Blood samples were collected and the parameters such as RBS, urea, creatinine, Na+, K+, Ca2+ were carried out by TBA 120 FR Autoanalyser. The eGFR was calculated using MDRD equation taking serum creatinine, age and sex of the patient. Haematocrit was measured by bioelectrical impedance method in percentage. Serum intact Parathyroid Hormone (iPTH) was measured using chemiluminiscence immuno assay by Beckmann Coulter. Red cell osmotic fragility was evaluated by incubating 50 μl of the RBC of the patients sample with gradually increasing concentrations of NaCl ranging from 0.1% to 0.9% [16]. After one hour, the incubated solutions were centrifuged and the absorbance of the supernatant was taken at 540 nm. A curve was plotted based on the concentration of NaCl and absorbance reading. The concentration of NaCl causing 50% haemolysis is taken as the Median Osmotic Fragility (MOF).
Statistical Analysis
Quantitative variables were expressed as Mean±SD. Linear regression and multi linear regression analysis were used to test the possible association between PTH, red cell osmotic fragility and eGFR. ROC curve analysis was done to determine the sensitivity and specificity of PTH in determining median osmotic fragility. Statistical analysis was done using SPSS version 21.0. Student’s t-test was applied to calculate the p-value and p-value<0.05 was considered to be statistically significant.
Results
The study revealed that 20 out of 40 CKD patients had Hb% level ≤ 10 (50%) in comparison seven out of 25 patients among the control group (28%). The rest of the biochemical parameters such as serum urea, creatinine, serum PTH also registered a higher value in the case group. Median osmotic fragility of RBC also revealed a significant rise in the CKD patients in comparison to the controls, i.e., 50% of RBC were lysed at 0.5±0.12 in comparison to 0.4±0.06 in the healthy controls [Table/Fig-1].
Baseline biochemical parameters of the cases and controls.
| Parameters | Controls (n=25)Mean±SD | Cases (n=40)Mean±SD |
|---|
| RBS (mg/dl) | 96.4±12.2 | 105.6±34.97 |
| Urea (mg/dl) | 46.0± 4.4 | 77.35±25.5* |
| Creatinine (mg/dl) | 1.4 ± 0.14 | 2.8±1.1* |
| Na+ (mmol/L) | 140.1 ± 4.2 | 138.9±7.8 |
| K+ (mmol/L) | 3.9± 0.15 | 4.5±0.6* |
| Ca2+ (mmol/L) | 1.08± 0.11 | 0.97±0.15* |
| PTH (pg/ml) | 47.0 ±10.6 | 179.8±80.5* |
| Hb (gm/dl)<10 | 7 (28%) | 20 (50%) |
| MOF (%) | 0.4 ±0.06 | 0.55±0.12* |
* p<0.05
[Table/Fig-2] depicted a significant fall in Hb% along with a rise in the serum PTH and MOF when the patients were stratified based on the estimated GFR values (eGFR).
Different parameters stratified according to stages of CKD.
| Parameters | Stage 2(n=2) | Stage 3A(n=18) | Stage 3B(n=15) | Stage 4(n=5) |
|---|
| eGFR(ml/min) | 65.8±1.56 | 42.3±8.5 | 19.8±4.7 | 11.2±2.44 |
| Hb%(gm%) | 13.75±0.21 | 10.12±1.43 | 9.24±1.29 | 7.98±1.36 |
| PTH(pg/ml) | 69.5±9.19 | 129.2±44.4 | 219.9±53.8 | 577.12±43 |
| MOF(%) | 0.35±0.12 | 0.48±0.11 | 0.62±0.07 | 0.65±0.66 |
*p<0.05
In [Table/Fig-3], multilinear regression analysis of MOF and other independent variables such as Hb%, Na+, Ca2+, K+, urea, PTH and creatinine highlighted the variance of MOF by 72% i.e., 72% of change in the MOF is contributed by combined effect of all these predictors where as [Table/Fig-4] revealed significant variation in MOF (74.7%) is contributed by unit change in PTH.
Multilinear regression analysis of MOF with combined effect of different predictors.
| Model | R | R2 | Adjusted R2 | Standard Error of the Estimate |
|---|
| 1 | 0.851a | 0.724 | 0.685 | 0.07277 |
Predictors: (Constant), HB%, Na+, Ca2+, K+, urea, PTH, creatinine
Dependent Variable: MOF
Multilinear regression analysis of MOF with individual effect of different predictors.
| Parameters | StandarizedCoefficient B | StandardError | t | p-value |
|---|
| PTH (pg/ml) | 0.747 | 0.000 | 4.909 | <0.001* |
| Urea (mg/dl) | 0.133 | 0.001 | 0.899 | 0.372 |
| Creatinine (mg/dl) | -0.089 | 0.015 | -0.548 | 0.586 |
| Na+ (mmol/L) | 0.087 | 0.001 | -1.170 | 0.247 |
| K+ (mmol/L) | -0.006 | 0.008 | -0.071 | 0.944 |
| Ca2+ (mmol/L) | 0.057 | 0.082 | 0.716 | 0.477 |
| Hb (gm/dl) | -0.127 | 0.006 | -0.978 | 0.332 |
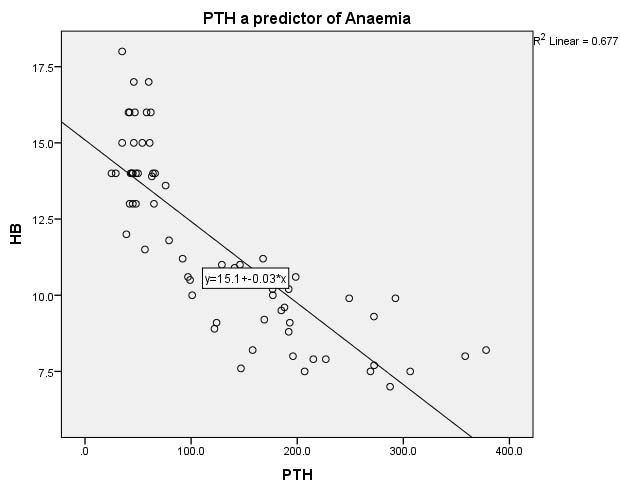
Linear regression of PTH with Hb% in [Table/Fig-5] reveals significant negative association between both the parameters with a R2 value of 0.677 i.e., 67.7% of the alteration in MOF is caused due to unit change in serum PTH.
Linear regression analysis of PTH with Hb%.
X-Axis-PTH in pg/ml, Y -Axis-Hb in gm/dl
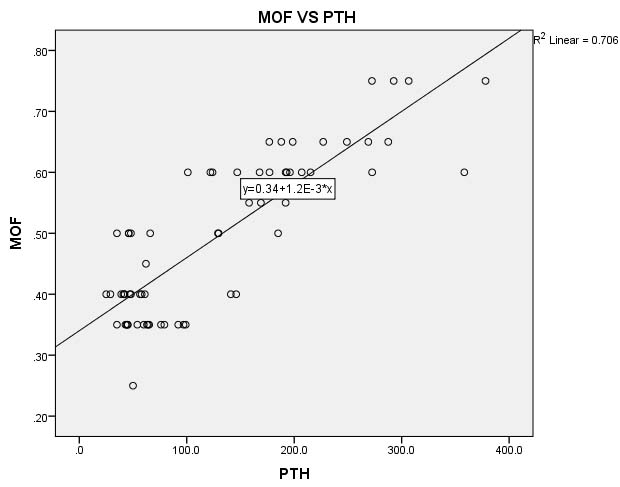
[Table/Fig-6] depicts the linear regression analysis of MOF with the PTH. MOF is the dependent variable and PTH is the independent variable and 70.6% of the variation of MOF is contributed by unit change in PTH.
Linear regression analysis of MOF with PTH.
X- Axis PTH in pg/ml, Y- Axis- Median Osmotic Fragility in %
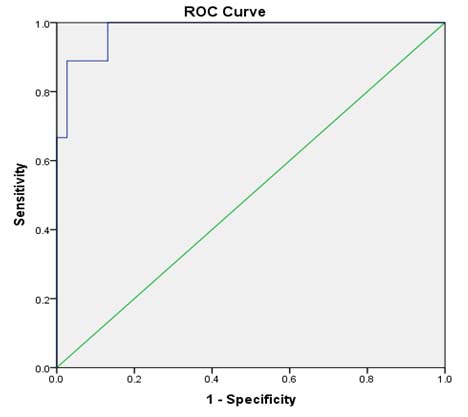
ROC analysis in [Table/Fig-7] revealed an area under the curve of 0.980 with a sensitivity of 100% and specificity of 87% in detecting osmotic fragility at a cut-off value of PTH ≥100 pg/ml.
ROC of PTH as diagnostic of osmotic fragility.
Discussion
Anaemia is one of the common complications in CKD patients associated with decline in the number of functional nephrons. Primary cause of anaemia is due to decrease in the erythropoetin production due to loss of renal functional mass [17]. Also, the impaired renal excretory function- leads to accumulation of toxin substances. Imbalance in the calcium phosphate, acid base and electrolytes resulting from impaired renal function affects the red cell shape and survival [18]. All these factors are responsible for low haemoglobin with declining kidney function. In our study, there was a significant fall in the haemoglobin level with a fall in the GFR [Table/Fig-2].
Parathyroid hormone is considered as uremic toxin [19]. Hyperparathyroidism secondary to CKD is caused due to underlying hyperphosphatemia, low calcium and calcitriol level. Anaemia has been recognized as a possible complication of hyperparathyroidism [7]. PTH is known to play a significant inhibitory role in RBC production and survival. Significant negative association was observed in between PTH and Hb% (R2=0.677, p<0.001). This is in line with other authors [20,21] who have identified that secondary hyperparathyroidism results in anaemia by various mechanisms such as, inhibiting erythropoesis, inducing marrow fibrosis and increase in the blood loss by reducing platelet aggregation.
In the present study, three patients with stage 1 attended the OPD for treatment, but they were unwilling for serum PTH assay and red cell fragility test. Hence, they were excluded from the study.
Median osmotic fragility revealed a significant rise with a fall in eGFR, [Table/Fig-2] highlighting the shortened survival of RBC with the progression of renal dysfunction. Erythrocyte survival is reduced in patients with advanced renal failure [22,23]. RBC from uraemic patients has normal survival when infused into normal subjects, whereas a shortened survival of RBC was observed when infused in uremic patients [22]. Several extra corpuscular factors may result in this reduced survival. Multilinear regression analysis revealed PTH to be the major factor responsible for the increased osmotic fragility of the RBC. The action of PTH on the osmotic fragility of the RBC is related to enhanced calcium entry into the cells [24]. The increased calcium influx inside the RBC leads to the stimulation of Ca2+ activated ATPase, which in turn results in ATP depletion and erythrocyte fragmentation. Increased PTH resulted in the increase in the Ca2+ concentration in the RBC leading to the cross-linking of membrane protiens. This affects the structure and stability of the red blood cells resulting in its lysis [25,26]. ROC curve analysis revealed an area under the curve of 0.980 in predicting osmotic fragility of RBC at a cut off value of PTH≥100 pg/ml which is highly significant. However, the extent to which PTH contributes to anaemia, remains controversial.
Limitation
The present study has several limitations which includes a smaller sample size, other factors such as serum Vitamin D, iron, ferritin were not estimated to find out the causal factors of anaemia and previous records of the haemoglobin status was not examined.
Conclusion
Secondary hyperparathyroidism, an unrecognised and untreated condition develops early in chronic kidney disease. Several studies have revealed the reverse association of anaemia with hyperparathyroidism. Mechanisms of anaemia due to Secondary Hyperthyroidism (SHPT) are unclear, severe bone marrow fibrosis with a concomitant reduction of space for erythrogenesis is an important cause of anaemia among these patients. This study reveals that renal secondary hyperparathyroidism has considerable effects on erythrocyte survival, contributing to increased fragility and anaemia. Emphasis should be laid on the early detection of serum PTH and the concomitant fall in haemoglobin levels should be treated, which in turn will help in slowing the progression of CKD and other associated comorbidites.
* p<0.05
*p<0.05
Predictors: (Constant), HB%, Na+, Ca2+, K+, urea, PTH, creatinine
Dependent Variable: MOF
[1]. Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Endogenous hormones and the risk of hip and vertebral fractures among older womenN Engl J Med 1998 339:733-38. [Google Scholar]
[2]. Gallieni M, Corsi C, Brancaccio D, Hyperparathyroidism and anaemia in renal failureAm J Nephrol 2000 20:89-96. [Google Scholar]
[3]. Kettler M, Petermann AT, Phosphate and FGF23 in early CKD: on how to tackle an invisible foeNephrol Dial Transplant 2011 26:2430-32. [Google Scholar]
[4]. DeBoer IH, Goerdetskaya I, Young B, Chertow GM, The severity of secondary hyperparathyroidism in chronic renal insuffiency in GFR dependent, race dependent and associated with cardiovascular diseaseJ Am Soc Nephrol 2002 13:2762-69. [Google Scholar]
[5]. Levin H, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Prevalance of abnormal serum Vitamin D, PTH, serum calcium and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney diseaseKidney Int 2007 71:31-38. [Google Scholar]
[6]. Fukagawa M, Kazama JJ, Kurokawa K, Renal osteodystrophy and secondary hyperparathyroidismNephrol Dial Transplant 2002 17(suppl 10):2-5. [Google Scholar]
[7]. Moe SM, Current issues in the management of secondary hyperparathyroidism and bone diseasePerit Dial Int 2001 21(suppl 3):S241-S46. [Google Scholar]
[8]. Ghaderian SB, Beladi Mousav S Si, Relationship between parathyroid hormone and anaemia in uremic patientsJournal of Parathyroid Disease 2014 2(1):39-40. [Google Scholar]
[9]. Bhadada SK, Bhansali A, Ahluwalia J, Chanukya GV, Behera A, Dutta P, Anaemia and marrow fibrosis in patients with primary hyperparathyroidism before and after curative parathyroidectomyClin Endocrinol (Oxf) 2009 70(4):527-32. [Google Scholar]
[10]. Smith LB, Fadrowski JJ, Howe CJ, Fivush BA, Neu AM, Furth SL, Secondary hyperparathyroidism and anaemia in children treated by hemodialysisAm J Kidney Dis 2010 55(2):326-34. [Google Scholar]
[11]. Chutia H, Ruram AA, Bhattacharyya H, Boruah P, Nath C, Association of secondary hyperparathyroidism with hemoglobin level in patients with chronic kidney diseaseJ Lab Physicians 2013 5(1):51-54. [Google Scholar]
[12]. Adhikary LP, Pokhrel A, Yadava SK, Khadka D, Thakur R, Relation between serum intact parathyroid hormone level and hematocrit in chronic kidney disease patientsKathmandu Univ Med J 2015 51(3):220-23. [Google Scholar]
[13]. KDIGO clinical practice guidelines for anaemia in chronic kidney diseaseKidney Int Suppl 2012 2:288 [Google Scholar]
[14]. Fernández-Rodríguez AM, Guindeo-Casasús MC, Molero-Labarta T, Domínguez-Cabrera C, Hortal-Casc n L, Pérez-Borges P, Diagnosis of iron deficiency in chronic renal failureAm J Kidney Dis 1999 34:508 [Google Scholar]
[15]. Rao DS, Shih MS, Mohini R, Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremiaN Engl J Med 1993 328:171-75. [Google Scholar]
[16]. Parpart AK, Lorenz PB, Parpart ER, Gregg JR, Chase AM, The osmotic resistance (fragilità) of human red cellsJ Clin Invest 1947 26:636-40. [Google Scholar]
[17]. Massry SG, Glassock RJ, Massry and Glassock’s Textbook of Nephrology 2001 4th edPhiladelphia, PALippincott, Williams & Wilkins [Google Scholar]
[18]. Christopher Mary M, Review of human loss and erythrocyte survival: uremia and anaemia in chronic kidney diseaseIsrael Journal of Veternary Medicine 2008 63(1):2008 [Google Scholar]
[19]. Drueke TB, Eckardt KU, Role of secondary hyperparathyroidism in erythropoietin resistance of chronic renal failure patientsNephrol Dial Transplant 2002 17(5):28-31. [Google Scholar]
[20]. Tutal E, Sezer S, Afsar B, Arat Z, Ozdemir FN, Haberal M, Additional effects of hyperparathyroidism on inflammatory status and rHuEpo requirements in hemodialysis patientsTransplantation Proceedings 2006 38(9):2807-12. [Google Scholar]
[21]. Baradaran A, Nasri H, Intensification of anaemia by secondary hyperparathyroidism in haemodialysis patientsMed J Islam Acad Sci 2001 14:161-66. [Google Scholar]
[22]. Brancaccio D, Cozzolino M, Gallieni M, Hyperparathyroidism and anaemia in uraemic subjects: a combined therapeutic approachJ Am Soc Nephrol 2004 15:S21-24. [Google Scholar]
[23]. Coen G, Calabria S, Bellinghieri G, Pecchini F, Conte F, Chiappini MG, Parathyroidectomy in chronic renal failure: short and long term results on parathyroid function, blood pressure and anaemiaNephron 2001 88:149-55. [Google Scholar]
[24]. Solidati L, Adano D, Zerbi S, Caumo A, Spaventa R, Bianchi G, Erythrocyte voltage dependent calcium influx is reduced in haemodialyzed patientsKidney Int 1999 56:190-97. [Google Scholar]
[25]. Agroyannis B, Kopelias I, Fourtounas C, Paraskevopoulos A, Fzanatos H, Dalamangas A, Relation between echinocytosis and erythrocyte calcium content in haemodialyzed uraemic patientsArtif Organs 2001 25:486-90. [Google Scholar]
[26]. Jonkisz DK, Purzyc L, Szczachor XL, Musial K, Danuta Z, The endogenous modulators of Ca2+, Mg2+ dependent ATPase in children in chronic kidney diseaseNephrol Dial Transplant 2010 25:438-44. [Google Scholar]