Azathioprine in Chronic Recalcitrant Erythema Nodosum Leprosum: A Case Report
Singh Shailendra Vikram Jitendra1, Romita Bachaspatimayum2, A. Subhalakshmi Devi3, S. Rita4
1 Postgraduate Student, Department of Pharmacology, Regional Institute of Medical Sciences, Imphal, Manipur, India.
2 Assistant Professor, Department of Dermatology, Venereology and Leprosy, Regional Institute of Medical Sciences, Imphal, Manipur, India.
3 Professor, Department of Pharmacology, Regional Institute of Medical Sciences, Imphal, Manipur, India.
4 Professor, Department of Pharmacology, Regional Institute of Medical Sciences, Imphal, Manipur, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Singh Shailendra Vikram Jitendra, Room No. 102, First floor, Gents Hostel No. 5, RIMS Campus, RIMS, Lamphelpat., Imphal-795004, Manipur, India.
E-mail: shailendravikram121@gmail.com
Erythema Nodosum Leprosum (ENL) may have a chronic course which may be recalcitrant to treatment. Preferred treatment modalities are systemic corticosteroids and thalidomide. Azathioprine, methotrexate and cyclosporine are immunosuppressants which may also be used as a steroid sparing agent. We report the case of a 48-year-old male diagnosed as lepromatous leprosy that developed ENL after four months of Multibacillary Multi-Drug Therapy (MB-MDT). He was treated with oral prednisolone (1 mg/kg/day) which was gradually tapered upto a dose of 7.5 mg/day. He developed recurrences on and off once the dose reached the said level and this continued for three years. Oral clofazamine (300 mg/day x 6 months; then 100 mg/day x 6 months) was also added in the second year. Thalidomide (200 mg/day) was also given but withdrawn due to adverse effect after 10 days. Azathioprine was started at a dose of 100 mg/day following which there was resolution of symptoms by one week and no recurrences by 10 weeks; it was given for eight months after which the dose was tapered to 50 mg/day for another four months. Complete withdrawal of oral prednisolone after gradual tapering was possible by 12 months of azathioprine therapy. The patient is still on regular follow-up with no recurrences so far till the last check-up.
Corticosteroid, Lepra reaction, Lepromatous leprosy
Case Report
A 48-year-old male patient came to the Outpatient Department (OPD) of the Department of Dermatology, Venereology and Leprosy, RIMS, Imphal, Manipur, India. with the complaints of multiple skin eruptions over the face, trunk and limbs of three months duration four years back when he first visited us and was diagnosed as a case of lepromatous leprosy. It was of gradual onset and slowly progressive in nature. There were no associated neurological symptoms. Cutaneous examination revealed multiple, discrete, shiny, non-tender, skin coloured papules and nodules of variable sizes (>0.5 cm x 0.5 cm - 1 cm x 1 cm) predominantly over the trunk [Table/Fig-1] and limbs with diffuse, mild induration over the forehead and bilateral ears. Skin biopsy revealed diffuse sheets of histiocytes with Fite Faraco stain showing multiple globi [Table/Fig-2]. Slit skin smear was positive with multiple globi and rod shaped bacilli. He was diagnosed as lepromatous leprosy and MB-MDT was started after routine investigations and test for glucose-6-phosphate dehydrogenase were done, which were within normal limits. There was significant reduction in the size of the lesions by three weeks. However, after four months, patient reported with recurrent eruption of painful reddish swellings over the skin which appeared in crops and subsided spontaneously associated with mild fever, myalgia, swelling of the face, hands and feet associated with tingling and numbness over the extremities. On examination, patient was afebrile with oedema over the face, hands and feet along with multiple, erythematous, tender, nodules over the trunk and extremities [Table/Fig-3]. There was mild reduction in sensation over the medial aspect of left big toe. Skin biopsy showed lymphomononuclear cells infiltrate in perivascular and periappendageal region with fibroblastic proliferation in the upper dermis only. He was diagnosed as having Type 2 lepra reaction or ENL and was started on oral prednisolone 1 mg/kg/day which was gradually tapered after significant improvement of the lesions which occurred by the second week. But when the dose reached 5 mg/day, the crops of nodules used to reappear and the patient had to be under a maintenance dose of 7.5 mg/day until a period of three years. During this period, he was also given oral clofazimine 300 mg/day for six months and 100 mg/day for another six months. Routine monitoring of haemogram, blood sugar, serum electrolytes along with liver and renal function tests were done which were within normal limits. MB-MDT was however withdrawn after completion of one year as advised from the State Leprosy Officer (SLO) as the drug was procured from the office of the SLO. Thalidomide 200 mg/day was tried after proper counseling of the patient but the patient reported after 10 days with facial and pedal oedema along with tingling and numbness of the extremities. The drug was withheld and he was given symptomatic treatment; routine haemogram and urine examination including liver and renal function tests were normal. After five days, patient’s condition improved and he was started on oral azathioprine 100 mg/day after due counseling so as to withdraw the systemic steroid. He was still given 7.5 mg/day of oral prednisolone. Thio- Purine Methyl Transferase (TPMT) test was not done due to non-availability. Routine haemogram and liver function tests were done two weekly and then monthly which were all normal. By eight weeks, the dose of steroid was reduced to 5 mg/day and gradually tapered off. Azathioprine was also reduced to 50 mg/ day after eight months and stopped after four months. The patient is still on regular follow-up and there have been neither any recurrences nor any adverse reactions to the drug till the last visit [Table/Fig-4].
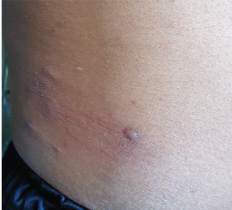
Shiny skin coloured and mildly erythematous papules, nodules and plaque over trunk.
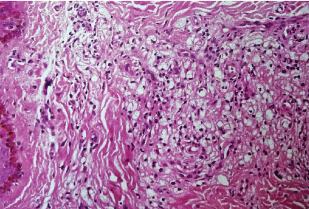
HPE (Haematoxylin & Eosin, 10x) showing diffuse granuloma suggestive of lepromatous leprosy.
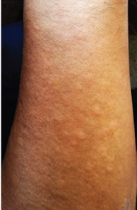
Erythematous nodule over left forearm.
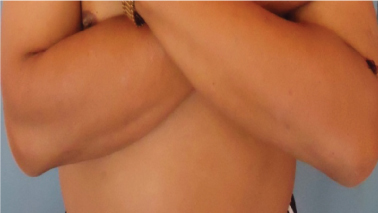
Last follow up: no recurrence of ENL lesions after azathioprine therapy.
Discussion
Leprosy reactions are immunological phenomena that may occur before, during or after the completion of MDT. Early diagnosis and treatment is required to prevent nerve function impairment and permanent disability [1]. MDT kills intracellular mycobacteria, which break up and release antigens thereby forming the focus for the formation of the immune complexes that cause ENL or Type 2 lepra reactions. Regular MDT may result in continued lysis and antigen availability [2]. Individuals with chronic recurrent ENL occurring for many years are at risk of developing complications secondary to prolonged corticosteroid therapy and may require alternative steroid sparing agents [3].
Since M. leprae infects peripheral nerves, the inflammation that is associated with reactions is a medical emergency, as severe nerve injury may develop rapidly, with subsequent loss of sensation, paralysis, and deformity. Type 2 reactions occur in multibacillary patients (lepromatous leprosy, LL and borderline lepromatous, BL) presenting as an abrupt onset of crops of very tender, erythematous nodules over the face, extremities or trunk. It may be associated with fever, malaise, and some degree of neuritis with sensory and motor neuropathy along with other complications like iridocyclitis and episcleritis, orchitis, arthritis, and myositis. It usually gets resolved in one to two weeks, but multiple recurrences may occur lasting for several months [4]. For severe ENL, prednisolone and clofazimine are most commonly used. Prednisolone usually acts rapidly by controlling the acute inflammation and relieving the pain, fever, and other signs [5]. Thalidomide is a highly effective drug in acute severe ENL. It causes rapid improvement in clinical symptoms with reduction in TNF-α level and also acts on T-cells, reducing the CD4 cells and increasing the CD8 cell counts. Serious limitations to its use arise due to its teratogenic effects and potential neurotoxicity [6] as had happened in our case. Azathioprine is a thiopurine immunosuppressant drug used in the management of many autoimmune and inflammatory skin diseases. Its parent drug 6-Mercaptopurine (6-MP), and the closely related 6-Thioguanine (6-TG), were originally developed for their anticancer properties; however, thiopurines are now more widely used for their anti-inflammatory and immunosuppressant effects [7].
Clofazimine and corticosteroids are used in the management of cases with severe ENL who are not responding satisfactorily to treatment with corticosteroids or where the risk of toxicity with corticosteroids is high [8]. Azathioprine and methotrexate along with prednisolone have been used for treatment of Type 2 reactions and may offer a steroid sparing regimen for treatment [9]. In one study, azathioprine was found to be a good adjuvant immunosuppressive drug in the control of recalcitrant Type 2 reactions, and especially as a steroid sparing agent with minimum side effects [10]. In our patient also, azathioprine helped not only in controlling the reaction but also in weaning the patient off the corticosteroid. Recommended treatment for recurrent or chronic ENL are a combination of clofazimine (300 mg/day for 3 months, 200 mg/day for 3 months and 100 mg/day as long as symptoms persists) plus prednisolone (30 mg/day for 2 weeks tapered by 5 mg every 2 weeks till 5 mg/day for 2 weeks and then stopped) or thalidomide (200 mg bd for 3-7days followed by 300 mg/day for 4 weeks, 200 mg/day for 4 weeks, 100 mg/day for 4 weeks and 100 mg on alternate days for 8-12 weeks) [11]. A cochrane review found some evidence of benefit for thalidomide and clofazimine, but generally no clear benefits for interventions in the management of ENL. Current guidelines for the management of ENL are given by bodies such as the World Health Organization (WHO) and the International Federation of Anti-Leprosy Associations (ILEP), however these guidelines are not supported by evidence from randomized controlled trials and are developed from practice [5]. In our patient, ENL persisted despite treatment with oral corticosteroid and clofazamine and steroid could be weaned off only after addition of oral azathioprine with no adverse effects neither recurrences till the last follow-up.
Conclusion
ENL reactions may have a prolonged course even with treatment with oral corticosteroids which may have serious complications if not controlled promptly. Azathioprine appeared to be a safe steroid sparing immunosuppressant as seen in our case. It may be given initially along with the corticosteroid for rapid control of the reaction after which the corticosteroid may be gradually withdrawn. Pharmacotherapeutic studies on a larger scale may be helpful to document its role in the control of ENL reactions.
[1]. Kahawita IP, Walker SL, Lockwood DNJ, Leprosy type 1 reactions and erythema nodosum leprosumAn Bras Dermatol 2008 83(1):75-82. [Google Scholar]
[2]. Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, IndiaAm J Trop Med Hyg 2006 74(5):868-79. [Google Scholar]
[3]. Walker SL, Waters MFR, Lockwood DNJ, The role of thalidomide in the management of erythema nodosum leprosumLepr Rev 2007 78:197-215. [Google Scholar]
[4]. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL, The continuing challenges of leprosyClin Microbiol Rev 2006 19(2):338-81. [Google Scholar]
[5]. Van Veen NH, Lockwood DN, Van Brakel WH, Ramirez Jr J, Richardus JH, Interventions for erythema nodosum leprosum. A Cochrane reviewLepr Rev 2009 80:355-72. [Google Scholar]
[6]. Verma KK, Srivastava P, Minz A, Verma K, Role of azathioprine in preventing recurrences in a patient of recurrent erythema nodosum leprosumLepr Rev 2006 77:225-29. [Google Scholar]
[7]. Meggitt SJ, Anstey AV, Mustapa MFM, Reynolds NJ, Wakelin S, British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprineBr J Dermatol 2011 165:711-34. [Google Scholar]
[8]. Radhakrishnan V, Vasumathi G, Ramalingam A, Sujatha SM, A case of septic shock- erythema nodosum leprosumSMJ 2015 2(3):15-18. [Google Scholar]
[9]. Mahajan VK, Sharma NL, Sharma A, Pulse dexamethasone, oral steroids and azathioprine in the management of erythema nodosum leprosumLepr Rev 2003 74:171-74. [Google Scholar]
[10]. Chaudhary R, Modi K, Azathioprine as a steroid sparing agent in recalcitrant Type 2 reactionIJSR 2014 4(4):1885-90. [Google Scholar]
[11]. Kar HK, Sharma P, Management of leprosy reactions. In: Kar HK, Kumar B, editorsIAL textbook of leprosy 2010 1st edNew DelhiJP Brothers Medical Publishers (P) Ltd:386-99. [Google Scholar]