Hepatitis B Core Antibody Negativity in a Chronic Hepatitis B Infected Patient: Report of an Unusual Serological Pattern
Vijeta Bajpai1, Ekta Gupta2, Naveen Kundu3, Shvetank Sharma4, SM Shashtry5
1 Senior Resident, Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India.
2 Additional Professor, Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India.
3 Senior Resident, Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India.
4 Assistant Professor, Department of Clinical Research, Institute of Liver and Biliary Sciences, New Delhi, India.
5 Assistant Professor, Department of Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ekta Gupta, Additional Professor, Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi-110070, India.
E-mail: ektagaurisha@gmail.com
Diagnosis of Hepatitis B Virus (HBV) infection is established by the presence of various hepatitis B serological and molecular markers. Expression of these serological markers may vary in natural course of HBV infection. We report a case of an unusual HBV serological pattern in a Chronic Hepatitis B (CHB) infected patient demonstrating absence of Hepatitis B core Antibody (Anti-HBc) in spite of presence of Hepatitis B surface Antigen (HBsAg) and HBV DNA. Since, anti-HBc represents a reliable serological marker for past exposure of HBV infection, therefore we emphasize on the presence of such unusual serological pattern which could lead to doubts in the interpretation of results.
Atypical serology, Diagnostic escape, Hepatitis B core antigen, Hepatitis B virus, Immunotolerance
Case Report
A 34-year-old man, known case of HBV infection with no history of alcoholism or smoking, admitted to liver care centre with continuous high grade fever (103°F) with chills, nausea and vomiting for the past 10-12 days. He had yellow discolouration of the eyes, mild upper abdominal pain and bilateral pedal oedema for seven days. On generalised physical examination, patient was deeply icteric. Abdominal examination revealed a soft, tender liver, palpable 4 cm below the costal margin. Ultrasonography of the whole abdomen was done that suggested cirrhotic changes in liver with moderate ascites. Liver histopathology finding showed moderate to severe inflammation with ground glass cytoplasm and numerous foci of lobular inflammation suggestive of chronic hepatitis. Biochemical investigations revealed mild leukocytosis. Liver function tests were markedly deranged and serum aminotransferases were more than two times the upper limit of normal [Table/Fig-1]. Blood sample was received in the virology laboratory and tested for various serological markers of HBV by Chemiluminescent Microparticle Immunoassay (CMIA) (ARCHITECT i2000SR Immunoassay Analyser, Abbott Diagnostics, Germany). It was positive for Hepatitis B surface Antigen (HBsAg) and negative for Hepatitis B core Antibody (Anti-HBc). To rule out false negativity, anti-HBc test was repeated with second Enzyme Linked Immunosorbent Assay (ELISA) (Anti-HBc Monolisa PLUS; Bio-Rad, France) and the results were in congruence. Other virological markers such as Hepatitis B envelope Antigen (HBeAg) was reactive; and Antibody to HBeAg (Anti-HBe), Antibody to Hepatitis B core IgM (Anti-HBc IgM) and Antibody to Hepatitis B surface Ag (Anti-HBs) were non-reactive. Other markers for acute hepatitis infection such as Hepatitis A Virus (HAV) IgM antibody and Hepatitis E Virus (HEV) IgM antibody were negative.
Baseline biochemical parameters of patient at the time of admission.
| Biochemical Parameters (Normal Range) | Values |
|---|
| Hb* (13-17 g/dL) | 12.3 |
| TLC (4000-11000/mm3) | 18.7 |
| Platelets (150x103-400x103/mm3) | 87 x103 |
| INR (2-3) | 2.18 |
| Serum-Bilirubin (Total/Direct/Indirect)(0.3-1.2/0-0.2/0.2-0.8 mg/dL) | 22/11.6/10.4 |
| AST/ALT (5-40/10-40 IU/mL) | 136/15 |
| SAP/GGT (32-92/7-64 IU/mL) | 119/68 |
| Albumin/Globulin (3.5-5.2/2-3.5 mg/dL) | 1.8/3.8 |
Hb*-Hemoglobin for men, TLC-Total Leukocyte Count, INR- International Normalized Ratio, AST/ALT- Aspartate Aminotransferase/Alanine Aminotransferase, SAP/GGT- Serum Alkaline Phosphatase/Gamma Glutamyl Transferase
Quantification of HBV DNA was done by real-time Polymerase Chain Reaction (PCR) using COBAS® TaqMan® 48 Analyser (Roche Molecular Diagnostics, Germany). The assay has Lower Limit Of Detection (LLOD) <6.0 IU/mL with a linear range 29 IU/mL to 1.1x108 IU/mL. In the present case, HBV DNA was found to be more than 1.1x 108 IU/mL in baseline blood sample. Follow up blood samples were collected from the patient on 5th, 10th and 15th days of admission and anti-HBc and anti-HBc IgM tests were repeatedly negative. Patient died on 17th day of post-admission because of severe coagulopathy and disseminated intravascular coagulation.
Amplification of precore/core region of the DNA was done by using in house design primers. [Table/Fig-2]. Nested PCR product (528 base pair) was then subjected to direct sequencing by the DNA Taq Dye Deoxy Terminator Cycle Sequencing Kit and ABI Prism 377 (Perkin Elmer Applied Biosystems, Foster City, CA). The sequencing results were blasted in National Center for Biotechnology Information (NCBI) site and analysis showed that the isolate was HBV genotype D subtype ayw2. Further, the sequences were compared with standard available sequences of genotype D and variations were noted [Table/Fig-3,4 and 5].
Description of surface and precore/core gene primers.
| Primer name | Surface gene primerPrecore/core gene primers |
| Sequence | S1-F: 5’-CATCAGGATTCCTAGGACCCCT-3’S3-R: 5’-AGGACAAACGGGCAACATAC-3’C1-F: 5’-TCACCTCTGCCTAATCATC-3’C3-R: 5’-GAGGGAGTTCTTCTTCTAGG-3’ |
| Nucleotide position* | S1-F (168–189), S3-R (458–478), C1-F (1825–1843), C3-R (2371–2391) |
| Amplicon size in base pair | (S)-269 bp, (C)-528 bp |
Surface gene primer (S), Precore/core gene primers (C), Forward primer (F), Reverse primer (R), Base pair (bp)
* S1-F (168–189) –Denoting nucleotide position extending from 168 to 189 in forward primer of surface gene, S3-R (458-478) –Denoting nucleotide position extending from 458 to 478 in reverse primer of surface gene, C1-F (1825–1843)- Denoting nucleotide position extending from 1825 to 1843 in forward primer of core gene, C3-R (2371–2391)–Denoting nucleotide position extending from 2371 to 2391 in reverse primer of core gene.
Substitutions in bases and core protein amino acid changes seen in sequence analysis.
| Substitutions seen in the isolate | Amino acid changes in core protein |
|---|
| G2011A | 37 |
| A2092T | 64 |
| G2138A, C2139T | 80 |
| C2242T | 114 |
| A2320G | 140 |
G-Guanine, A-Adenine, T –Thymine, C-Cytosine
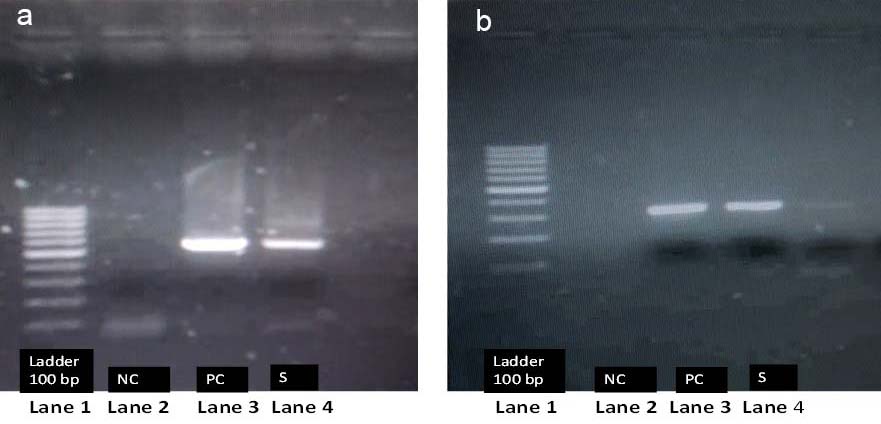
Agarose gel electrophoresis of nested PCR products: a) Showing bands of precore/core gene product (269bp); b) Showing bands of surface gene product (528bp).
Lane 1: Ladder (100 bp)
Lane 2: Negative Control (NC)=Molecular grade water
Lane 3: Positive Control (PC)=Previously known positive sample of HBV positive patient
Lane 4: Patient Sample (S)
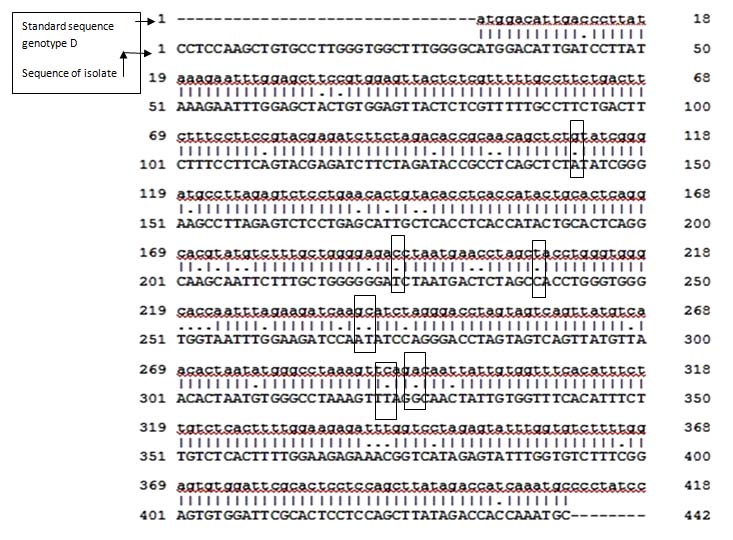
Sequence analysis of precore/core region and comparison with standard genotype D.
Nucleotide in small letter (a, t, g, c) represents sequences of standard genotype D (1-418). Nucleotide in capital letter (A, T, G, C) represents sequences of our isolate genotype D (subtype ayw2) (1-442).
Dots (.) represent substitution position between nucleotide. Box represents positions of various nucleotide substitutions which are mentioned in [Table/Fig-3] (G2011A, A2092T, G2138A, C2139T, C2242T, A2320G) which can result in to amino acid change.
Discussion
We report an interesting case of absence of anti-HBc in spite of presence of HBsAg and other HBV markers. Anti-HBc is one of the sensitive and commonly used marker for detection of past HBV exposure in clinical laboratories and blood banks [1,2]. It is detected in patient’s serum after 3–4 weeks of appearance of HBsAg and usually persists for many years irrespective of HBV infection outcome [3]. Anti-HBc is formed against Hepatitis B core Antigen (HBcAg) which is considered "particulate" and it does not circulate in the blood. HBcAg induces a vigorous humoral and cellular immune response resulting in production of anti-HBc that may persist for lifetime [4].
In this case report, various probable explanations for absence of anti-HBc were considered; first of all, it could have been due to false negativity of the analytical assay [5]. We ruled out this possibility by confirming anti-HBc negativity by a second assay utilizing different principle. Second possible cause for this serological pattern could have been due to immunodeficiency state of the patient, like seen in patients on anti-cancer treatment or on immunosuppression etc., [6]. But our patient was not in immunodeficiency state. Third mechanism for anti-HBc antibody negativity could be any variations or substitutions in the core region of the HBV genome, which could have lead to production of altered antibodies that were not detected by routine assay [7].
In our case, it could be predicted that substitutions in various positions (G2011A, A2092T, G2138A, C2139T, C2242T, A2320G) in core promoter gene had resulted in production of altered and truncated HBcAg protein. These substitutions could potentially have contributed to abnormal immune reaction and negativity for anti-HBc. Since, HBcAg is the principal target of the strong immunogenic response in host as well as for various detection assays, the mutations in this protein inhibit core nucleocapsid formation and produce defects in HBcAg assembly [8]. These mutations can also result in altered expression of HBcAg, prolonged inflammation of liver, but had never been reported with anti-HBc negativity [9]. Thus, an abnormal immune reaction and an imbalance in the production of pro-inflammatory and anti-inflammatory cytokines contribute to the outcome of acute exacerbation of HBV and poor prognosis of patients [10].
Fourth and one of the probable causes of anti-HBc negativity is found in individuals born to HBsAg and HBeAg positive mothers [11]. This is because of tolerogenic effect of antigens (HBeAg and HBcAg) transmitted to children from mother during birth. These antigens act as tolerogenic proteins and establish a T helper cell induced immunotolerance effect and result in anti-HBc negativity. In a study done by Pondé RA, it has been discussed that phenomenon of the immune tolerance to HBcAg has been associated with the vertical transmission and inhibits anti-HBc antibody production [12]. In our case, the patient’s mother was positive for HBsAg but her HBeAg status could not recognised from patient records. Therefore, we cannot strongly suggest that only this phenomenon of immunotolerance is responsible for anti-HBc negativity in our case. Further, if long term follow up of the patient could have been possible, we could have probably excluded the delayed immune response of anti-HBc antibody.
Conclusion
Absence of detectable anti-HBc in serum of the patient could be due to diagnostic escape mutants in core gene of HBV and probably because of immunotolerance to HBcAg. Further investigations are required to be done to diagnose such mutants in early stages of infection.
Hb*-Hemoglobin for men, TLC-Total Leukocyte Count, INR- International Normalized Ratio, AST/ALT- Aspartate Aminotransferase/Alanine Aminotransferase, SAP/GGT- Serum Alkaline Phosphatase/Gamma Glutamyl Transferase
Surface gene primer (S), Precore/core gene primers (C), Forward primer (F), Reverse primer (R), Base pair (bp)
* S1-F (168–189) –Denoting nucleotide position extending from 168 to 189 in forward primer of surface gene, S3-R (458-478) –Denoting nucleotide position extending from 458 to 478 in reverse primer of surface gene, C1-F (1825–1843)- Denoting nucleotide position extending from 1825 to 1843 in forward primer of core gene, C3-R (2371–2391)–Denoting nucleotide position extending from 2371 to 2391 in reverse primer of core gene.
G-Guanine, A-Adenine, T –Thymine, C-Cytosine
[1]. Krajden M, McNab G, Petric M, The laboratory diagnosis of hepatitis B virusCan J Infect Dis Med Microbiol 2005 16:65-72. [Google Scholar]
[2]. Gessoni G, Beggio S, Barin P, Favarato M, Galli C, Valverde S, Significance of anti-HBc only in blood donors: a serological and virological study after hepatitis B vaccinationBlood Transfus 2014 12:63-68. [Google Scholar]
[3]. Liang TJ, Hepatitis B: the virus and diseaseHepatology 2009 49:13-21. [Google Scholar]
[4]. Cao T, Lazdina U, Desombere I, Hepatitis B virus core antigen binds and activates naive human B cells in vivo: Studies with a human PBL-NOD/SCID mouse modelJ Virol 2001 75:6359-66. [Google Scholar]
[5]. Bendinelli M, Pistello M, Freer G, Vatteroni M, Maggi F, Rose NR, Manual of Clinical Laboratory Immunology 2002 Washington DCASM Press:696-717. [Google Scholar]
[6]. Avettand-fenoel V, Thabut D, Katlama C, Immune suppression as the etiology of failure to detect anti-HBc antibodies in patients with chronic hepatitis B virus infectionJ Clin Microbiol 2006 44(6):2250-53. [Google Scholar]
[7]. Alexopoulou A, Mutants in the precore, core promoter, and core region of Hepatitis B virus, and their clinical relevanceAnn Gastroenterol 2009 22:13-23. [Google Scholar]
[8]. Kim H, Seoung-Ae Lee, Yeon S, Kim BJ, Precore/core region mutations of hepatitis B virus related to clinical severityWorld J Gastroenterol 2016 22:4287-96. [Google Scholar]
[9]. Hu F, Bi S, Yan H, Shi Y, Shen J, Associations between hepatitis B virus basal core promoter/pre-core region mutations and the risk of acute-on-chronic liver failure: a meta-analysisVirol J 2015 12:87 [Google Scholar]
[10]. Ren X, Xu Z, Liu Y, Li X, Bai S, Ding N, Hepatitis B virus genotype and basal core promoter/precore mutations are associated with hepatitis B-related acute-on-chronic liver failure without pre-existing liver cirrhosisJ Viral Hepatitis 2010 17:887-95. [Google Scholar]
[11]. Chen M, Sällberg M, Hughe J, Jones J, Guidotti LG, Chisari FV, Immune tolerance split between Hepatitis B virus precore and core proteinsJ Virol 2005 79(5):3016-27. [Google Scholar]
[12]. Pondé RA, Atypical serological profiles in hepatitis B virus infectionEur J Clin Microbiol Infect Dis 2013 32(4):461-76. [Google Scholar]