Disseminated Burkitt’s Lymphoma with a Pancreatic Mass in a HIV Positive Woman Diagnosed by Axillary Lymph Node Biopsy
Godfrey Mutashambara Rwegerera1, Yordanka Pina Rivera2, Feng Zhou3, Kudra Jumanne Chobanga4, Sheikh Omar Sesay5
1 Senior Lecturer, Department of Internal Medicine, Faculty of Medicine, University of Botswana and Princess Marina Hospital, Gaborone, Botswana.
2 Lecturer, Department of Internal Medicine, Faculty of Medicine, University of Botswana and Princess Marina Hospital, Gaborone, Botswana.
3 Pathologist, Department of Histopathology, National Health Laboratory Gaborone, Botswana.
4 Lecturer, Department of Obstetrics and Gynecology, Faculty of Medicine, University of Botswana and Princess Marina Hospital, Gaborone, Botswana.
5 Consultant, Department of Radiology, Princess Marina Hospital, Gaborone, Botswana.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Godfrey Mutashambara Rwegerera, Private Bag 00713, 4775 Notwane Road. Gaborone, Botswana.
E-mail: grwege@yahoo.com
Burkitt’s Lymphoma (BL) is a highly aggressive B cell lymphoma of non-Hodgkin’s type usually presenting in extranodal sites for endemic and sporadic types of the disease. Like other non-Hodgkin’s Lymphomas (NHL), HIV positive associated BL is associated with peripheral lymphadenopathy. We present a case of 22-year-old newly diagnosed HIV positive female patient who presented with generalized peripheral lymphadenopathy and obstructive jaundice. Initial work up was suggestive of acute pancreatitis with further evaluation revealing a pancreatic head mass. BL was confirmed both by axillary lymph node biopsy and immunohistochemistry, highlighting the importance of high index of suspicion and prompt histopathological diagnosis to enable treatment of this fatal disease that is potentially curable.
Epstein-barr virus, Fatal outcome, Immunodeficiency, Young adult
Case Report
A 22-year-old female, newly diagnosed HIV positive who was not yet placed on antiretroviral medications was referred to Princess Marina Hospital, a tertiary center in Gaborone, the capital city of Botswana. She had presented at a local hospital with history of right upper quadrant abdominal pain since one month which worsened with time. She was noted to have sclera jaundice. The abdominal pain severe with high intensity at the hypogastic region radiating to the back. She admitted to have a history of fever associated with occasional chills, nausea, non-projectile vomiting post feed and loss of appetite with loss of 8 kilograms weight in the span of six weeks. Initial assessment at the referral hospital revealed a chronically ill looking lady, cachexic, pale with deep sclera icterus, she had generalized peripheral lymphadenopathy involving cervical, axillary and inguinal regions; most of the lymph nodes were palpable and were rubbery in consistency. She was febrile at 38.30C with pulse rate of 122 beats per minute, Blood pressure of 99/50mmHg and respiratory rate of 18 breaths per minute and 100% saturation on room air. Systemic examination was significant for abdominal findings with tender hepatomegaly of 6 cm below right costal margin and liver span of 14 cm. Other systems including nervous, cardiovascular and respiratory were unremarkable. Patient baseline blood, urine, culture and sensitivity results were as shown in [Table/Fig-1]; they revealed leukocytosis, normocytic anaemia and liver enzymes showed an obstructive picture and excisional biopsy sample was obtained from the left axillary gland and sent to National Health laboratory for diagnosis. Abdominal pelvic ultrasound revealed hepatomegaly of 16.5 cm, with hypoechoic nodules noted in the right middle lobe, measuring 52.1 x 47.7 x 17.4 mm. The pancreas was diffusely enlarged with hypoechoic texture; enlarged lymphnodes in the porta-hepatis, paraaortic and inguinal regions were also observed, with the largest lymphnode at right inguinal region measuring 61.6 x 45.6 mm. Initial chest radiography was normal without any evidence of cardiomegaly, hilar adenopathy or pleural effusion. Computed Tomography (CT) scan of the abdomen performed on day 4, post-admission revealed moderate ascites, hepatomegaly with enlarged intrahepatic ducts. The pancreas was diffusely enlarged with pancreatic head mass measuring 7.74 x 5.61 cm [Table/Fig-2] and mixed densities of 14.3 HU to 60 HU post contrast with moderate ascites [Table/Fig-2]. The common bile duct was grossly enlarged. Both kidneys were normal in shape, position and size. Generalized lymphadenopathy involving peri-pancreatic, porta-hepatis, para-aortic [Table/Fig-2] and para-caval regions were also observed. There were multiple hepatic masses and periportal nodes [Table/Fig-2] and the spleen appeared normal. CT chest was significant for bilateral pleural effusion without mediastinal or hilar adenopathy. Histology results of the left axillary nodes were as follows; Gross findings of the excisional biopsy from the axillary lymph node revealed two ovoid grey brown encapsulated masses; one 3.5 x 3 x 2.4 cm and the other 3.8 x 3 x 1.8 cm. All cut-sections appeared yellow and soft with foci of haemorrhage and necrosis. Microscopy revealed a starry-sky pattern impacted by interspersed tangible-body macrophages [Table/Fig-3]; there was diffuse infiltrate of medium-sized malignant lymphoid cells with round nuclei, coarse chromatin, two to three basophilic nucleoli and scanty cytoplasm [Table/Fig-4]. Frequent mitoses, foci apoptotic bodies and haemorrhage were also seen. The histological features were consistent with a high grade NHL, favoring BL. Immunohistochemistry of the tumor cells showed diffuse nuclear PAX-5 positivity, CD10 and CD20 positivity, 100% KI 67 expression and Epstein-Barr Virus (EBV) in-situ hybridization was also positive [Table/Fig-5] whereas BCL6, BCL2, CD3 and MUM1 were negative [Table/Fig-6]. The conclusion was made of high grade NHL of burkitt’s type and oncology review was sought urgently. Over the course of admission patient received supportive care that included analgesia, dietary support, intravenous fluids and three packs of blood transfusion Patient condition worsened over the course of admission with new onset worsening shortness of breath saturating at 80-88% on oxygen therapy; this was despite therapeutic thoracocentesis. Oncology assessment was done and since patient was critically sick so was not fit for chemotherapy. Patient died on day 12 of admission and next of kin did not consent to autopsy.
Laboratory investigations of the patient.
| Laboratory investigation | Patient results | Reference value |
|---|
| WBC | 16.95 | 4-11 |
| Hb | 6.4 g/dl | 12-15 |
| MCV | 81.2 fl | 83-99 |
| PLT | 646 x 103/μL | 150-400 x 103 |
| Serum creatinine | 56 μmol/ l | 50-99 |
| Urea | 4.2 μmol/ l | 2.9-7.1 |
| Serum Na+ | 133 μmol /l | 135-145 |
| Serum K+ | 5.0 μmol /l | 3.5-5.1 |
| Alkaline phosphatase (ALP) | >1853 U/l | 35-129 |
| Gamma-Glutamyltranspeptidase (GGT) | 1239 U/l | 11-50 |
| Alanine aminotransferase (ALT) | 280 U/l | 11-41 |
| Aspartate aminotransferase (AST) | 446 U/l | 10-34 |
| Total bilirubin | 169 μmol/l | 1-25.7 |
| Conjugated bilirubin | 153 μmol/l | 0-3 |
| Lactate dehydrogenase (LDH) | 2055 U/l | 60-160 |
| Hepatitis B surface antigen | Negative | |
| Hepatitis C antibodies | Negative | |
| Urine culture and sensitivity | No growth | |
| Blood culture and sensitivity | No growth after seven days | |
| Beta HCG | Negative | |
| Alpha feto protein | 2.4 ng/dl | 0-20 |
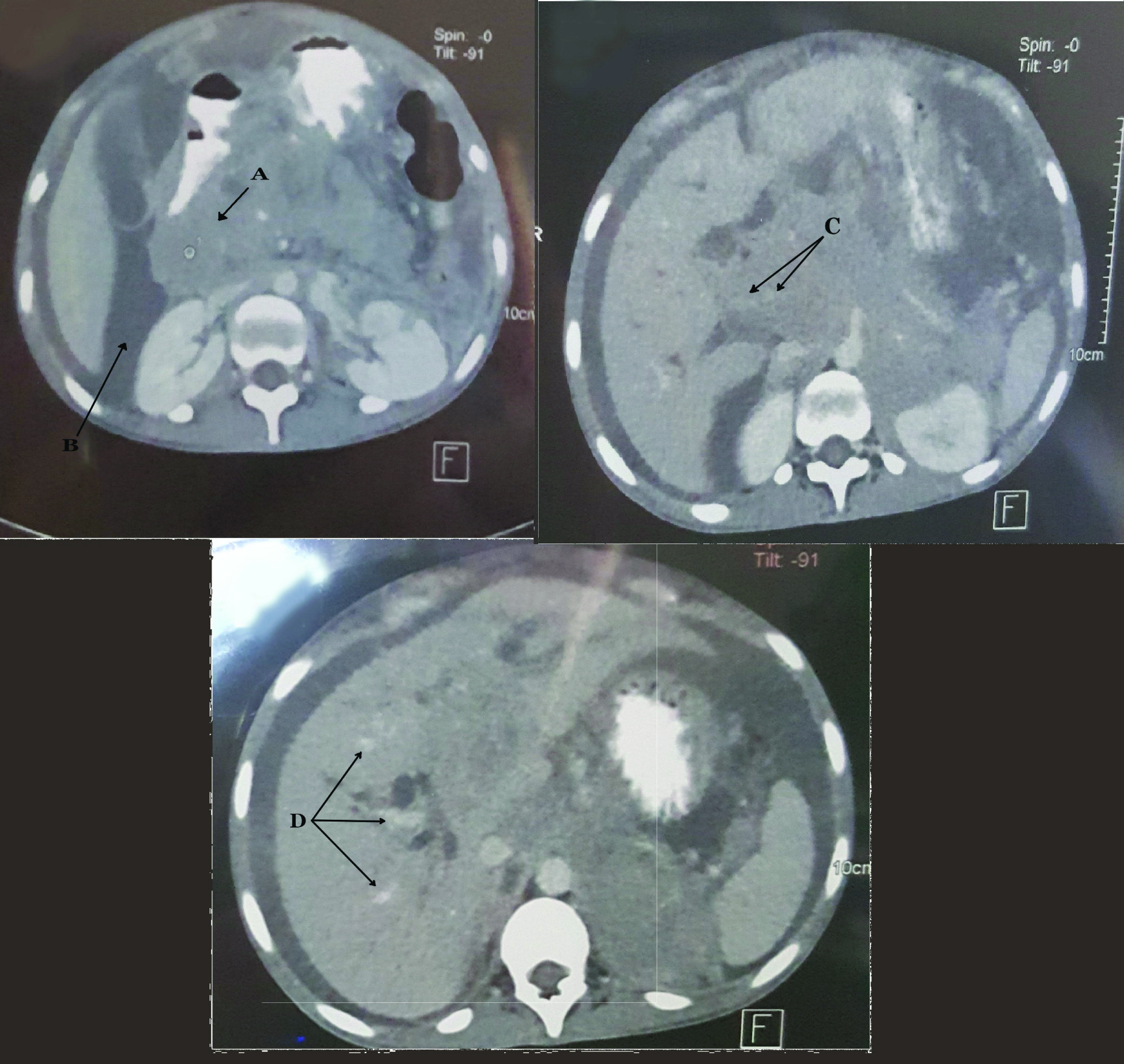
Findings are consistent with diffuse enlarged pancreas with pancreatic head mass (a) and ascites (b); peri-pancreatic lymph nodes (c) and multiple hepatic masses and porta-hepatic nodes (d).
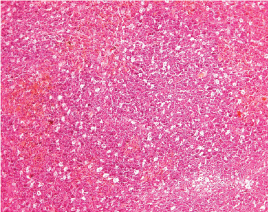
Biopsy showing a monotonous lymphoid diffuse infiltrate with a starry-sky appearance (10x10; H and E stain).
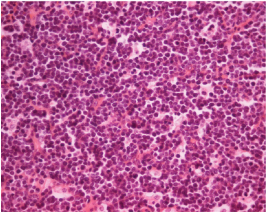
Biopsy showing medium sized neoplastic cells with round nuclei, coarse chromatin, multiple distinct nucleoli and frequent mitoses (10 x 40; H and E stain).
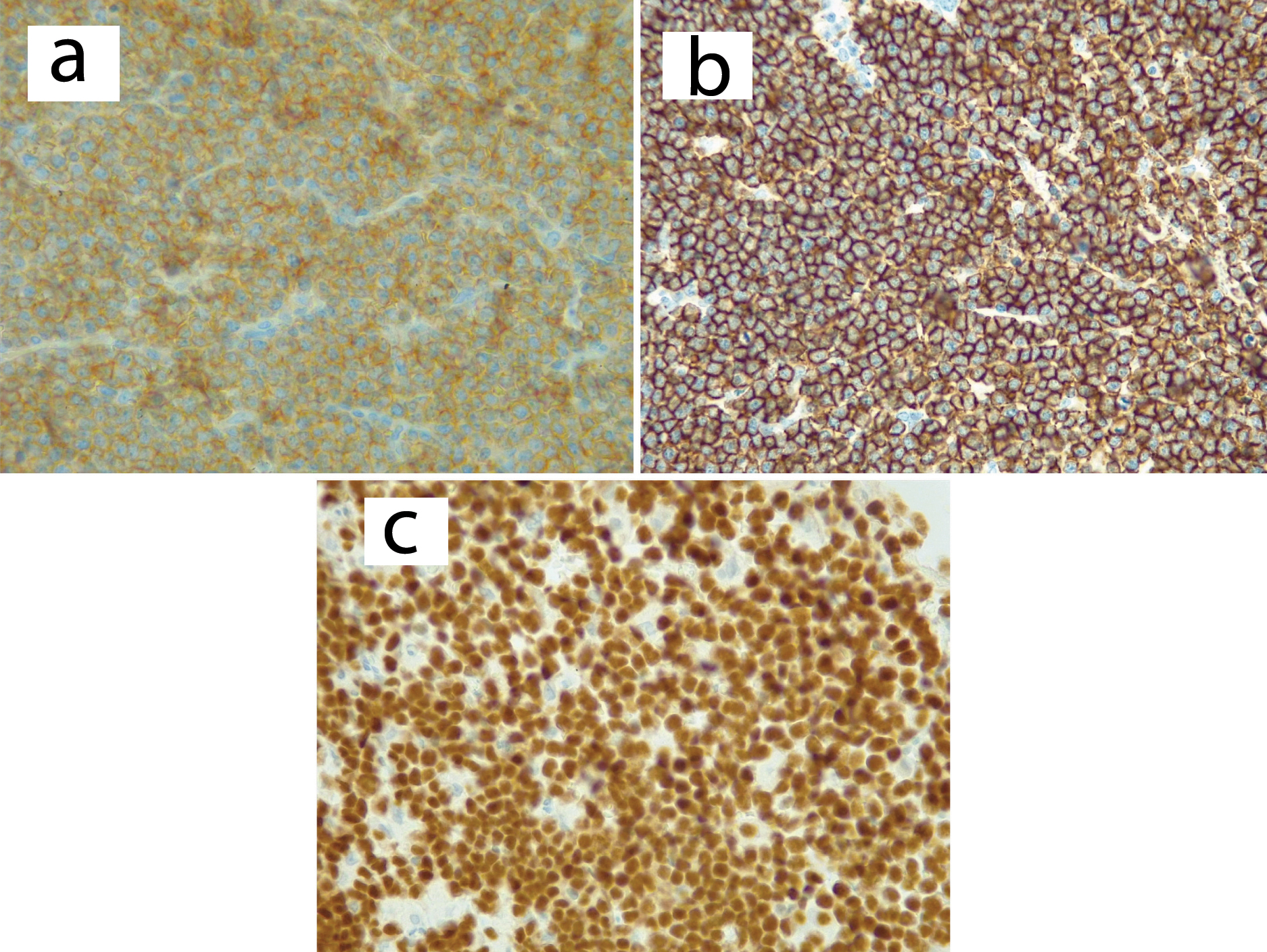
Positive immunohistochemistry stains for CD10 at 40X (a), CD20 (b), PAX-5 (c), KI 67 expression (d) and EBV positive in-situ hybridization (e).
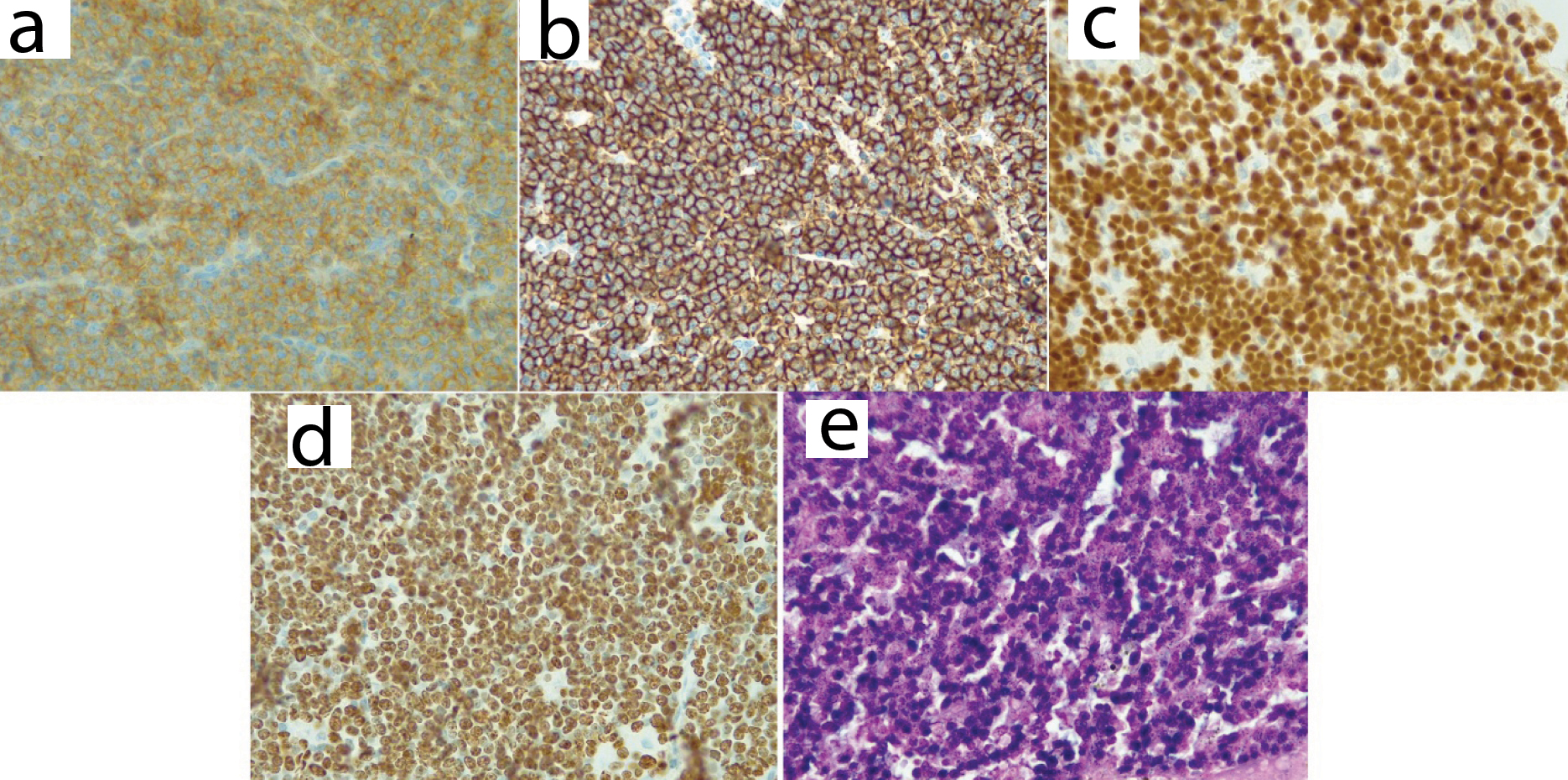
Negative immunohistochemistry stains for BCL-2 at 40X (a); BCL-6 (b); and MUM1 (c).
Discussion
BL is a highly aggressive B type of NHL in human beings with highest proliferation rate and potential to double the number of tumor cells within 24 hours [1]. It has three main varieties; endemic BL, sporadic BL and immunodeficiency-associated BL [2,3]. Acquired Immunodeficiency Syndrome (AIDS), congenital immunodeficiency syndromes and patients with allograft transplants comprises the immunodeficiency category [4]. The pathogenetic hallmark of BL is rearrangement of the c-Myc oncogene which is an important regulator of cell proliferation and apoptosis [5]. The typical reciprocal chromosomal translocations in BL include: t (8; 14) (q24; q32) or their rare variants: t (8; 22) (q24; q11) or t (2; 8) (q12; q24) [6,7]. The pathogenesis of HIV-associated BL is linked to oncogenic virus co-infection with about 40% association with EBV. EBV cause chronic HIV antigen stimulation that provokes expansion of polyclonal B-cells [8]. The patient presented in this case report was positive for EBV as it was revealed by EBV in-situ hybridization.
Our patient presented with advanced disease with generalized peripheral lymphadenopathy, multiple hepatic masses and involvement of multiple lymph node regions in the abdomen. Involvement of pancreas either as diffuse or with a pancreatic mass as it occurred in this case is rare [9,10]. Initial ultrasonagraphy was consistent with acute pancreatitis; this is similar to the previous reported cases [9,11]. On the other hand, BL is typically associated with extranodal disease in immunocompetent individuals [12]; immunodeficiency related Burkitt’s lymphoma has been documented to involve lymph nodes [13] as it occurred in this case providing room for less invasive diagnostic modality; furthermore mediastinal lymph nodes are always spared in BL [13], as it occurred in our patient.
Involement of pancreas in lymphoma poses a challenge of distinguishing origin as to whether lymphoma is of pancreatic origin or diffuse infiltration from adjacent lymph nodes; - this is due to the fact that the pancreas lacks a capsule [14]. Behrns KE et al., defined lymphoma of pancreatic origin as, a lymphoma localized to the pancreas, a pancreatic mass with involvement of peripancreatic lymph nodes without distal lymph node involvement, no hepatic or splenic metastasis and a normal white blood cell count. According to this definition, our case did not qualify as lymphoma of primary pancreatic origin because the distal lymph nodes were involved, white blood cell count was increased (leucocytosis) and hepatic involvement was seen. Thus it was more of a difuse infiltration instead of being lymphoma of primary pancreatic origin [15]. On the other hand, pancreatic involvement from adjacent lymph node involvement in NHL has been reported in up to 30% of cases and in most cases resembling acute pancreatitis as it appeared in our patient [9,11].
Our patient presented with obstructive jaundice; this is a very rare observation in NHL [16] as it has been reported only in about 1% of cases at presentation [12]. Obstructive jaundice usually occurs at the level of distal common bile duct and liver hilum secondary to compressing lymph nodes [17].
Use of immunohistochemistry has been found to be useful in confirming diagnosis of BL in cases where histology findings are not definitive. Presence of positive CD20, CD10 and 100% KI 67 expression confirm the diagnosis of BL [2,18,19]. The presented patient had both characteristic histology findings of macrophages depicting a “starry-sky” appearance [3,18] and described immunohistochemistry findings.
Conclusion
BL is a highly aggressive NHL with high response to chemotherapy when prompt diagnosis is made. HIV- associated BL tends to involve peripheral nodes providing room for less aggressive diagnostic approaches. Despite the fact that obstructive jaundice is a rare finding in NHL including BL, its presence should always alert a possibility of disseminated lymph nodes associated with lymphoma.
[1]. Kemp S, Gallagher G, Kabani S, Noonan V, O’Hara C, Oral non- Hodgkin’s lymphoma: review of the literature and World Health Organization classification with reference to 40 casesOral Surg Oral Med Oral Pathol Oral Radiol Endod 2008 105(2):194-201. [Google Scholar]
[2]. Sangma MM, Dasiah SD, Ashok AJ, Ileo-colic Burkitt lymphoma in a young adult female-a case reportJ Clin and Diagn Res 2016 10(4):11-12. [Google Scholar]
[3]. Patankar S, Venkatraman P, Sridharan G, Kane S, Burkitt’s lymphoma of maxillary gingiva: A case reportWorld J Clin Cases 2015 3(12):1011-16. [Google Scholar]
[4]. Biko DM, Anupindi SA, Hernandez A, Kersun L, Bellah R, Childhood Burkitt lymphoma: abdominal and pelvic imaging findingsAmerican Journal of Roentgenology 2009 192(5):1304-15. [Google Scholar]
[5]. Park YH, Kim WS, Kang HJ, Na II, Ryoo BY, Yang SH, Gastric Burkitt lymphoma is a distinct subtype that has superior outcomes to other types of Burkitt lymphoma/leukemiaAnn Hematol 2006 85(5):285-90. [Google Scholar]
[6]. Ugar DA, Bozkaya S, Karaca I, Tokman B, Pinarli FG, Childhood craniofacial Burkitt’s lymphoma presenting as maxillary swelling: Report of a case and review of literatureJ Dent Child (Chic) 2006 73:45-50. [Google Scholar]
[7]. Neville BW, Damm DD, Allen CM, Bouquot JE, Soft tissue tumors. In: Neville BW, Damm DD, Allen CM, Bouquot J, editorsOral and Maxillofacial pathology 2009 3rd edSt. LouisW.B. Saunders Elsevier:557-559. [Google Scholar]
[8]. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Burkitt’s LymphomaLancet 2012 379(9822):1234-44. [Google Scholar]
[9]. Mahajan V, Qian Y, Blake B, Rojas-Khalil Y, Radhakrishnan RS, Muthukumar A, Sporadic Burkitt lymphoma presenting as acute pancreatitis, concurrent sinusitis, and enlarged adenoidsCase Reports in Pediatrics 2016 2016:3862175 [Google Scholar]
[10]. Sağlam M, Yılmaz MI, Mas MR, Taşçı I, Örs F, Sönmez A, A case of pancreatic Burkitt lymphoma: radiological findingsDiagn Interv Radiol 2009 15(1):39-42. [Google Scholar]
[11]. Bacchi LM, Ucella I, Amancio TT, Gonçalves MC, Palhares RB, Siqueira SAC, Burkitt lymphoma mimicking acute pancreatitisAutopsy Case Rep [Internet] 2012 2(3):5-11. [Google Scholar]
[12]. Odemis B, Parlak E, Basar O, Yuksel O, Sahin B, Biliary tract obstruction secondary to malignant lymphoma: experience at a referral centerDig Dis Sci 2007 52(9):2323-32. [Google Scholar]
[13]. Kanbar AH, Burkitt Lymphoma and Burkitt-like Lymphoma Clinical Presentationhttp://emedicine.medscape.com/article/1447602-clinical. Last accessed on 15th April 2017 Updated on 4/8/2016 [Google Scholar]
[14]. Guermazi A, Brice AP, De Kerviler E, Fermé C, Hennequin C, Meignin V, Extranodal Hodgkin disease: spectrum of diseaseRadiographics 2001 21(1):161-79. [Google Scholar]
[15]. Behrns KE, Sarr MG, Strickler JG, Pancreatic lymphoma: is it a surgical disease? Pancreas 1994 9(5):662-67. [Google Scholar]
[16]. Chaudhari D, Khan S, Saleem A, Taylor T, Reddy C, Borthwick T, Obstructive jaundice as an initial manifestation of non-Hodgkin lymphoma: treatment dilemma and high mortalityCase Reports in Medicine 2013 (2013):259642 [Google Scholar]
[17]. Ravindra KV, Stringer MD, Prasad KR, Kinsey SE, Lodge JP, Non-Hodgkin lymphoma presenting with obstructive jaundiceBr J Surg 2003 90(7):845-49. [Google Scholar]
[18]. Kılınç A, Saruhan N, Tepecik T, Gündoğdu B, Burkitt’s lymphoma presenting as maxillary swelling: case reportMiddle Black Sea Journal of Health Science 2016 2(2):29-32. [Google Scholar]
[19]. Mehanna KH, Telles JEQ, Mauro DP, Burkitt’s lymphoma presenting as jejunojejunal intussus-ception in a child: A case reportInt J Case Rep Images 2017 8(2):96-100. [Google Scholar]