Endometrial carcinoma is the second most common gynecologic malignancy with an incidence of 5.9 per 100,000 women in the developing countries. In India, the incidence is 4.3 per 100,000 women [1]. EMAC are divided into two broad histologic types. Type 1 includes EMAC and mucinous carcinoma accounting for about 80% of the cases wherein there is unopposed estrogen stimulation, associated with precursor lesions such as Atypical Endometrial Hyperplasia (AEH)/Endometrial Intraepithelial Neoplasia (EIN), presenting with low tumour grade and showing distinct genetic abnormalities such as PTEN, PAX2and k-ras mutation. Type 2 includes USC, CCC, undifferentiated carcinoma and carcinosarcoma accounting for about 10% of the cases less associated with estrogen stimulation, presenting with higher tumour grade and stage. USC exhibit early TP53 mutations and serous intraepithelial carcinoma is proposed as its preinvasive precursor [2-4]. The peak age incidence for EH in western countries for simple and complex EH without atypia was 142/100,000 woman-years and 213/100,000 woman-years in early 50s and that of atypical hyperplasias was 56/100,000 woman-years in the early 60s [5]. According to recent 2014 WHO classification, hyperplasias are classified as hyperplasia without atypia and AEH/EIN [6] Hyperplasia without atypia do not show relevant genetic alterations and less than 2% progress to carcinoma in case endocrine abnormality persists. However, AEH progresses to EMAC in 23% of cases [2]. Molecular biomarkers that have shown independent prognostic value in endometrial cancer, focusing on survival and/or risk of lymph node metastases include TP53 mutation, loss of hormone receptors, and Ki67 [7]. In present study, we have attempted to study the expression pattern of ER, PR, p53 and Ki67 in EHs and endometrial carcinomas.
Materials and Methods
This retrospective study included 85 cases of EHs, 28 cases of endometrial carcinomas, total 113 cases, diagnosed and operated upon between Jan 2010 to Feb 2017. It was conducted in the Department of Pathology in Smt. Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India. Ethical clearance was obtained from the Institutes Ethical Clearance Committee. The tissues for histopathology (surgical specimens, dilatation and curettage) were received in the Department of Pathology over a period of seven years. All routinely processed paraffin embedded tissue blocks and Hematoxylin and Eosin (H&E) stained slides of these 113 cases were retrieved. The slides were reviewed. For EMAC, FIGO grading and staging was done. For EHs, subtypes were classified according to 2014 WHO classification [6].
A technique of manual tissue array was employed for all the cases subjected for IHC. The primary antibodies used were ERα (Clone EP1; Dako), PR (Clone PgR636; Dako), p53 (Clone DO-7; Dako) and Ki-67 (Clone MIB-1; Dako). Negative control was included in all batches. Section from normal breast tissue was used as positive control for ER and PR. A section from prostate and tonsil was used as positive control for p53 and Ki67 respectively. Sections were examined under High Power Field (HPF) [8].
The evaluation of ER and PR was performed according to the method described by Carcangiu ML et al., based on the percentage of stained cells and the intensity of nuclear stain [2]. The percentage of positive cells was graded as follows: 1, 0%s to 25% of the nuclei stained; 2, 26% to 75% of nuclei stained; 3, more than 76% of the nuclei stained. The staining intensity was scored as follows: 1, absent or weak; 2, strong; and 3, very strong. The sum of both parameters gave the immunohistochemical score. Tumours were divided into three categories depending on the immunohistochemical score. Category I corresponded to a score of 2, Category II to a score of 3 or 4, and Category III to a score of 5 or 6. Category I tumours were considered as immunonegative, whereas Category II and III tumours were considered as immunopositive [Table/Fig-1,2]. The reaction for p53 was recorded as percentage of tumour cells showing nuclear staining with p53. Tumour cells with p53>2% were considered positive [9]. The nuclear staining for Ki67 was graded by counting Ki67 labelling Index (Ki67LI). Ki67LI was recorded as percentage of positively stained tumour nuclei in 1000 tumour cells in the hot spot of tumour. Tumour cells with Ki67LI of 5% or more were considered positive [10].
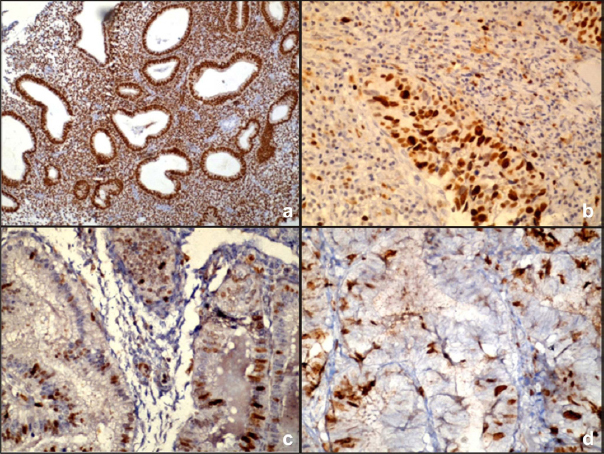
a) ER expression in simple endometrial hyperplasia without atypia (4X) (ER% 2+ ER staining intensity 3=5 category III); b) PR expression in simple endometrial hyperplasia with atypia (40X) (PR% 2+ PR staining intensity 3=5 category III); c) p53 expression in complex endometrial hyperplasia without atypia (40X) (p53=1%, negative); d) Ki67 expression in complex endometrial hyperplasia with atypia (40X) (ki67= 12%).
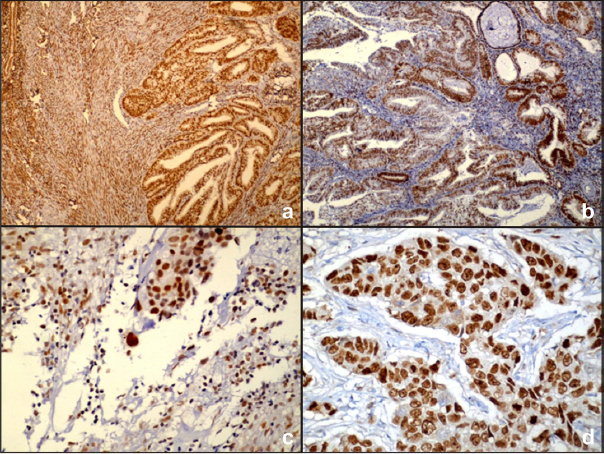
a) ER expression in Grade I endometrioid adenocarcinoma (4X) (ER% 2+ ER staining intensity 1=3 category II); b) PR expression in Grade II endometrioid adenocarcinoma (4X) (PR% 1+ PR staining intensity 2=3 category II); c) p53 expression in uterine serous carcinoma (40X)(p53=25%); d) Ki67 expression in clear cell carcinoma (40X) (ki67=50%).
Statistical Analysis
The statistical software named Primer software Version 5.0 (manufactured by McGraw-Hill) was used for analyses of the data. The groups were compared using the Pearson’s Chi-square test (PCT). ANOVA test was used to compare the difference in mean between multiple groups. The t-test was used to compare the difference in mean between two groups. The p-value of 0.05 or less were considered statistically significant.
Results
Distribution of various types of EH and endometrial carcinomas according to histopathological types in this study is shown in [Table/Fig-3].
Distribution of various types of endometrial hyperplasia and endometrial adenocarcinoma according to histopathological subtypes.
| Lesion type | No. of cases | Percentage |
|---|
| Endometrial hyperplasia without atypia | 70 | 61.95 |
| Atypical endometrial hyperplasia/ endometrial intraepithelial neoplasia | 15 | 13.27 |
| Endometrioid adenocarcinoma | 22 | 19.47 |
| Uterine serous carcinoma | 2 | 1.77 |
| Clear cell carcinoma | 4 | 3.54 |
| Total | 113 | 100 |
Clinical Findings
The peak incidence of EH was seen in 41-50 years. Mean age was 44.52±7.3 years. The youngest patient of EH was 26-year-old, premenopausal and oldest patient was 70-year-old, presenting with postmenopausal bleeding both diagnosed as Simple Endometrial Hyperplasia (SEH) without atypia on histopathology. The peak incidence of endometrial carcinomas was seen in 51-60 years of age. Mean age was 58.14±9.57 years. Youngest patient was 40-year-old, diagnosed as EMAC Grade I on histopathology. The oldest patient was 80 years of age, diagnosed as CCC on histopathology. About 52.21% cases in our study were postmenopausal (59/113), this was followed by premenopausal (30.97%, 35/113) and the rest were perimenopausal (16.81%, 19/113). The commonest chief complaint with which the patients in our study presented with was postmenopausal vaginal bleeding (52.21%, 59/113) followed by menorrhagia (44.25%, 50/113). Nearly 3.54% of the patients in our study had other complaints like infertility and polymenorrhea.
Grading
We applied modified FIGO grading system for grading of EMAC. Out of 22 cases of EMAC, 50% (11/22) cases were Grade I, 40.91% cases were Grade II (9/22) and the rest were Grade III (9.09, 2/22). The non EMACs in our study were CCC and USC. Out of 15 cases of EMACs received as hysterectomy specimens, 12 cases were of Stage IB, one each case was of Stage IA, IIB and IIIC. 12 cases among 15 cases of EMAC, showed less than half of myometrial invasion. One case was Stage IA i.e., limited to the endometrium. Rest two cases showed myometrial invasion involving more than half of myometrium. Among 15 cases of EMACs that were received as hysterectomy specimen, only one case showed presence of adenomyosis.
IHC
[Table/Fig-4,5] shows the IHC expression of EHs and endometrial carcinomas. [Table/Fig-6] shows comparison of IHC expression with grades of EMAC. Comparison of IHC expression with age in cases of EMACs is shown in [Table/Fig-7].
ER, PR, p53 and Ki67 expression in endometrial hyperplasia and endometrial carcinoma.
| Lesion Type (Number) | ER | PR | p53 | Ki67 | Ki67LI |
|---|
| Endometrial hyperplasia without atypia (70) | 65 (92.86%) | 63 (90%) | 6 (8.57%) | 53 (75.71%) | 8.4% |
| Atypical endometrial Hyperplasia/ Endometrial intraepithelial neoplasia (15) | 13 (86.67%) | 13 (86.67%) | 8 (53.33%) | 13 (86.67%) | 9.8% |
| Endometrioid adenocarcinoma (22) | 17 (77.27%) | 18 (81.81%) | 21 (95.45%) | 22 (100%) | 27.5 % |
| Uterine serous carcinoma (2) | 0 | 0 | 2 (100%) | 2 (100%) | 47.5% |
| Clear cell carcinoma (4) | 0 | 0 | 4 (100%) | 4 (100%) | 48% |
ER, PR, p53 and Ki67 expression in endometrial hyperplasia and endometrial carcinoma.
| Lesion | ER | PR | p53 | Ki67 |
|---|
| Endometrial hyperplasias (85) | 78 (91.76%) | 76 (89.41%) | 14 (16.47%) | 66 (77.65%) |
| Endometrial carcinomas (28) | 17 (60.71%) | 18 (64.28%) | 27 (96.43%) | 28 (100%) |
| Chi square test | χ2=12.933;p<0.01 | χ2=7.795;p<0.01 | χ2=54.838;p<0.01 | χ2=6.011;p<0.05 |
Comparison of ER, PR, p53 and Ki67 expression with grades of endometrioid adenocarcinoma.
| Grade | ER | PR | p53 | Ki67 | Ki67LI |
|---|
| Grade I (n=11) | 9 (81.82%) | 9 (81.82%) | 10 (90.91%) | 11 (100%) | 22.52% |
| Grade II (n=9) | 8 (88.89%) | 9 (100%) | 9 (100%) | 9 (100%) | 30.55% |
| Grade III (n=2) | 2 (100%) | 0 | 2 (100%) | 2 (100%) | 40% |
Chi square test (χ2=3.53; p=0.740)
Comparison of expression of ER, PR, p53 and Ki67 markers and age of the patient in endometrioid adenocarcinoma.
| Age (years) | ER | PR | p53 | Ki67 | Mean Ki67LI |
|---|
| 31-40 (n=1) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 20% |
| 41-50(n=1) | 1 (100%) | 1 (100%) | 0 | 1 (100%) | 25% |
| 51-60(n=15) | 11 (73.33%) | 12 (80%) | 15 (100%) | 15 (100%) | 33% |
| 61-70(n=3) | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 25% |
| 71-80(n=2) | 1 (50%) | 1 (50%) | 2 (100%) | 2 (100%) | 35% |
Discussion
Endometrial carcinoma is the most common malignancy of the female genital tract [1]. Time honored prognostic factors include patient’s age, tumour grade, stage, histologic type, and the depth of myometrial invasion. Various studies have investigated the endometrial immunomarkers which could directly affect prognostication [6].
In the present study, 85 cases of EHs and 28 cases of endometrial carcinomas were distributed in the age range of 26 to 80 years. It could be observed that peak incidence of EH was in fifth decade and that of endometrial carcinomas was in sixth decade. The age wise distribution of EHs and endometrial carcinomas was comparable with previous studies [11-13]. This could be justified by the suggestion that epithelial transformation from the benign to the malignant may develop over a time period by progressive increase in the degree of abnormality.
In the present study, ER expression was seen more in EH without atypia (65/70, 92.85%) than in AEH (85.71, 12/14) and endometrial carcinoma (17/28, 60.71%). This was statistically significant (χ2=15.357; p<0.01). PR expression was seen more in EH without atypia (63/70, 90%) than in AEH (12/14, 85.71%) and endometrial carcinoma (18/28, 64.28%). This was statistically significant (χ2=9.470; p<0.01). This shows that ER and PR expression has inverse correlation with the severity of endometrial lesion. This is parallel to the studies in literature [14-16].
In present study, ER and PR expression was similar except in two cases of EH without atypia where ER was positive PR was negative and one case of EMAC where ER was negative and PR was positive. Few recent studies have documented absence of PR expression associated with worse prognosis [17]. In the present study, one case of EMAC where ER was negative and PR was positive presented as Stage IB and Grade 2.
In the present study, ER and PR expression was absent in all Grade III EMAC (2/2), CCC (2/2) and USC (4/4) as reported in most studies [18]. ER and PR expression was most seen in Grade II EMACs. It is documented in literature that neither ER nor PR expression correlates with stage, myometrial invasion, or lymph node metastasis. However, many recent reports suggest that positive expression of PR correlates with low tumour grade, low recurrence rate, and higher survival [17]. In the present study, 13/15 cases of ER and 10/15 cases of PR were expressed in Stage I EMAC. Also, 10/15 cases of ER and 11/15 cases of PR were expressed in EMAC showing less than half of myometrial involvement. No Stage II (1/15) and Stage III (1/15) cases showed ER and PR expression.
A p53 expression increased as the severity of the endometrial lesion increased from EH to endometrial carcinoma and this was statistically significant (χ2=56.467; p<0.01). Ilie D et al., performed study on 30 diagnosed cases of EH and compared it with normal endometrium and endometrial carcinoma. Cases that showed p53 immunoreactivity belonged to Complex Endometrial Hyperplasia (CEH) (3 cases, 30%) and AEH (6 cases, 60%) [9]. All cases of endometrial carcinomas were p53 positive [18]. Boruban MC et al., in their study stated that p53 gene mutation was not found in EH, but researchers have detected this mutation in 20% of cases of endometrial carcinoma and 90% of cases of USC [18]. In our study, p53 expression increased as the grade of EMAC increased though statistically insignificant (χ2=5.304; p=0.071). Maximum EMAC showed p53 values ranging from 1% to 10%. Two cases showed p53 values of 20% and 25%. In CCC, p53 expression was found to range from 5% to 60%. A p53 values were 25% and 70% in two cases of USC. Lax SF et al., performed study on 21 CCC of endometrium and compared them with 77 EMAC of all grades and 30 USC. They found p53 expression tended to be higher in CCC compared with EMAC though statistically insignificant [19]. One study mentioned lower p53 expression for CCC as compared to USC [16]. In present study, two cases of CCC expressed higher p53 values of 50% and 60% corresponding with the CCC with serous features on histomorphology. Ki67 positivity increased as the severity of endometrial lesions increased from EH to endometrial carcinoma which was statistically significant (χ2=6.106; p<0.05). Mean Ki67LI increased from 8.4% in EH without atypia to 9.8% in AEH. This was statistically significant {F=28.02, p<0.01 (ANOVA)} [Table/Fig-8] Ki67 positivity was seen in all cases of EMAC with mean Ki67LI increasing from a value of 22.52 % to 40% as the grade increased. This was statistically significant {F=39.95, p<0.01 (ANOVA)} [Table/Fig-9]. Mean Ki67LI values were highest for USC and CCC in the present study. This is similar to the most studies in literature [8,16]. Zidan AA et al., in their study on 40 cases of EH and endometrial carcinoma found 10/40 cases (25%) were positive for Ki67. The expression increased from SEH (10%) and AEH (16.7%) to endometrial carcinoma (38.9%) [8]. Lax SF et al., performed study on 21 cases of endometrial CCC and compared them with 77 EMAC of all grades and 30 USC. They found the Ki67 proliferation index was significantly higher in CCC compared with EMAC [19]. Stoion SC et al., in their study on 22 cases of EMAC found proliferation index values ranged between 11% to 42%. They found that Ki67 proliferation index was highest in poorly differentiated carcinomas (39%), with the invasion of the external half of the myometrium (18%), and those in Stage III tumour (34%) [16].
Ki67 labelling Index in Endometrioid hyperplasia.
| Endometrial Hyperplasia (number of cases) | Ki67 labelling index (%) |
|---|
| Mean ± SD | Range |
|---|
| Endometrial hyperplasia without atypia (70) | 4.97±3.11 | 1-10 |
| Atypical endometrial Hyperplasia/ Endometrial intraepithelial neoplasia (15) | 9.6±2.89 | 3-15 |
F=28.02, p-value < 0 .01 (ANOVA)
Ki67 labelling Index in Endometrioid adenocarcinoma according to tumour grade.
| Endometrioid adenocarcinoma | Ki67 labelling index (%) |
|---|
| Grade | Mean ± SD | Range |
|---|
| I | 22.52±3.59 | 20-30 |
| II | 30.55±1.67 | 30-35 |
| III | 40±2.8 | 38-42 |
F=39.95, p-value < 0 .01 (ANOVA)
ER, PR, p53 and Ki67 are known to have a role in differentiating poorly differentiated EMAC (PD-EMAC) and USC as seen in the present study [3]. Focal nuclear ER and PR immunoreactivity was observed in PD-EMAC in comparison with USC. The p53 values were higher in USC (2, 47.5±31.81, 25-70) as compared to PD-EMAC (2, 6.5±4.94, 3-10). The difference in mean of p53 values between PD-EMAC and USC was statistically insignificant {F=3.24, p=0.213 (ANOVA)} [Table/Fig-9]. High p53 cut off value of about 25% would accurately differentiate all cases of EMAC (<25%) and USC (>25%) in the present study. The mean Ki67LI was higher in USC as compared to PD-EMAC [Table/Fig-8]. The difference in mean Ki67LI between PD-EMAC (2, 40±2.83, 38-42) and USC (2, 47.5±3.53, 45-50) was statistically insignificant (F=5.5, p= 0.144, ANOVA). A study with larger sample size is needed to elucidate the matter further. Thus, a panel of these four markers helps in arriving at a correct histopathological diagnosis. Markers such as vimentin, p16, HMGA2 are also been studied to solve the dilemma [3]. Recent studies have reported mixed EMAC and USC types which have molecular and epidemiological similarities to pure USC and thus are suggested to receive similar treatment as USC [20].
Limitation
Smaller sample size of endometrial carcinoma cases.
Conclusion
ER, PR expression decreased as the lesion advanced from EH without atypia to AEH, from EH to EMAC and from EMAC to CCC and USC. p53, Ki67 and mean Ki67LI expression increased from EH without atypia to AEH and was seen greater in endometrial carcinoma being higher for CCC and USC than EMAC. ER, PR was absent in Grade III and Stage II and III EMAC cases. The p53 and Ki67 expression and mean Ki67LI increased as the grade of EMAC increased. The findings of this study indicate that ER, PR status, p53 and Ki67 if included in each pathology report will pave the way for better understanding of biological behavior and may help tailor individual treatment strategies.
F=28.02, p-value < 0 .01 (ANOVA)
F=39.95, p-value < 0 .01 (ANOVA)