Excessive ROS production may promote tissue injury and inflammation in affected individuals. This accentuates immune suppression especially in those with weakened anti-oxidant capacity [1]. Co-infection of Human Immunodeficiency Virus (HIV) in TB patients further increases the generation of ROS [2,3]. The trans-activator of transcription (tat) protein of HIV leads to heightened production of ROS in HIV infected patients by generation of superoxide anion in the mitochondria. This in turn may activate Nuclear Factor κB (NF-κB) [4,5] thus increasing HIV transcription.
Under normal conditions during the cellular metabolism due to production of ROS like superoxide anion and hydrogen peroxide (H2O2) the lungs are exposed to the basal oxidative stress. In pulmonary TB, macrophages undergo respiratory burst after contact with the bacterium [6]. These cells possess the capacity to generate huge amounts of ROS which are not adequately removed and these ROS induces the Lipid Peroxidation (LP), rise in intracellular calcium ions and DNA damage [7,8].
Various studies have showed that changes in cellular redox status from a normal physiological state may provoke cellular responses such as proliferation or apoptosis or cell necrosis or changes in the DNA leading to mutational changes [9,10]. Exposure to these oxidants by the cellular systems and their responses create conditions that are favourable for viruses so they can replicate their life cycle such as HIV, Hepatitis C virus, etc., [11,12].
The present study compared the pro-oxidant and antioxidant levels as makers of oxidative stress and free radicals production in TB patients and TB patients co-infected with HIV infection.
Materials and Methods
In this prospective study all experiments were conducted on Human volunteers were approved by the Institutional Ethical Committee (IEC) of Seth GSM College and KEM Hospital vide letter no. EC/67/2011 dated 27th June 2011. The samples were collected from Department of Pathology, Seth GS Medical College and KEM Hospital, and Group of Tuberculosis Hospital, Sewree, Mumbai, India. The study was done at Department of Biochemistry and Virology Haffkine Institute, Parel, Mumbai, India.
Study Groups
The study population consisted of 50 HIV-TB seropositive patients and 50 pulmonary TB positive patients as determined by patients’ clinical history. Patients belonged to both gender and were above 18 years of age. Fifty healthy volunteers of either sex i.e., males and females from work site were included as controls. Sample size was chosen on the basis of some previously published similar studies [13-15].
The informed consent was taken from the entire study subject i.e., patients and control study subject before taking the blood samples from them. All the study subjects were informed about the study and possible benefits and risks if any while collecting the blood samples.
The patients were categorised using the National AIDS Control Organisation (NACO) and Revised National Tuberculosis Control Program (RNTCP) [16,17], guidelines for HIV and Tuberculosis. All the patients were enrolled on the HAART and AKT therapy. No drug naïve patients were included. All cases were confirmed HIV-TB positive and TB positive using the clinical records and case consultation with the clinicians at the respective hospital.
Control subjects were primarily screened on the basis of taking history whether they were not having any inflammatory conditions like infection of viral diseases in the past six to eight months. The informed consent was taken from each individual volunteering for the study.
Exclusion criteria: Individuals below 18-year-old and pregnant women were excluded from the study.
Blood Collection
Approximately, 10 ml of whole blood from the study population and healthy volunteers were collected in Ethylene Diamine Tetra Acetic Acid (EDTA) and plain vacutainers tubes. Serum was separated from plain vacutainers tube, aliquoted and stored at -80°C and used for the following assays.
Determination of Nitric Oxide (NO) [
18]
To determine the nitric oxide in the samples Griess’s reagent [5] method was used. About 1% sulphanilamide solution in 5% o-phosphoric acid and 0.1% N-(1-Naphthyl) ethylene diamine dihydrochloride (NED) solution was allowed to equilibrate to room temperature. About 50 μL of 1% sulphanilamide solution was added to 50 μL of standard/serum sample and to which 50 μL of 0.1% NED solution was added. The solution was mixed and the absorbance was taken at 520 nm.
Estimation of Thiobarbituric Acid Reactive Species (TBARS) [
19]
Lipid peroxides were estimated by measurement of TBARS by modified method of Brown and Kelly. About 50 μL of standard/ serum sample was added in amber glass vials containing 250 μL of 1.22 M o-phosphoric acid, 450 μL distilled water and 250 μL of TBA reagent. Tetramethoxy propane was used as standard. The mixture was incubated at 95°C in a water bath for 60 minutes. The samples were cooled on ice followed by addition of 360 μL methanol and 40 μL of 1 M NaOH to neutralize the samples. The absorbance was measured at 532 nm. Results obtained were expressed in nmole/mL for serum [20].
C-Reactive Protein (CRP)
Serum CRP was estimated using a commercially available kit (Tulip Diagnostics, Mumbai). According to the manufacturer instructions, 3 µL of calibrator and samples with 450 µL of buffer were added to tubes. Optical Density (OD 1) of standards, controls and samples was read at 546 nm. To this 50 µL of CRP antiserum was added; the solutions were mixed and incubated for five minutes at room temperature. Optical density (OD 2) of calibrator and samples were again measured at 546 nm. The O.D. was calculated using (i.e., OD 2- OD 1), CRP activity was measure in g/dL.
Superoxide anion was measured from Peripheral Blood Mononuclear Cells (PBMCs) using the Nitro Blue Tetrazolium (NBT) salt to an insoluble blue formazan. About, 100 microliters (μL) of 1 mg/mL NBT solution was added to 100 μL PBMC’s (cell count =1×106 cells). The cells were incubated overnight; following which the tubes were centrifuged at 1500 rpm for 15 minutes and the supernatant was discarded. The pellet was washed with PBS to remove the excess of medium. The cells were then treated with 120 μL of 2 N potassium hydroxide and 140 μL of Dimethyl Sulfoxide (DMSO). The absorbance of the supernatant was measured at 650 nm and the values were expressed in OD units.
Serum catalase activity was determined by the method described by Abei. Catalase activity was measured spectrophotometrically at 240 nm. The reaction mixture containing 2.9 mL of phosphate buffer pH 7.4 with 30 mM H2O2 and 10 μL of serum sample was added in the cuvettes. The reaction was measured for three minutes and the specific activity was expressed as units/mg of protein.
Superoxide Dismutase Activity [
23]
Superoxide dismutase activity was determined by the method described by Marklund and Marklund based on auto-oxidation of pyrogallol in alkaline solution. The reaction mixture containing 2 mL of Tris–cacodylate buffer pH 8.5 and 250 μL of sample was added and the reaction was started by addition of 30 mM pyrogallol. The rate of auto-oxidation was measured as the incremental difference in A420 (ΔO/D) for three minutes on UV spectrophotometer. A single unit of enzyme was expressed as 50% inhibition of pyrogallol and specific activity was expressed as units/mg of protein.
Total Serum Proteins
The total serum proteins were determined using the commercially available kit (Transasia Biomedical, Mumbai) following the Biuret method. The assay was performed as per the manufacturer instruction. The assay was carried into tubes containing 1000 μL of biuret reagent added into Blank, standard and test tubes. To this standard 20 μL was added in the tube, while 20 µl of test samples was added to the tubes and incubated for 10 minutes at 37°C. The absorbance of standard, and each test was measured at 546 nm. The serum total protein was expressed in g/dL.
Statistical Analysis
The results were expressed as Mean±SD. The statistical significance of the data was determined by one-way ANOVA test with Bonferroni comparison. Statistical analysis was done using Graph Pad Prism 5 Software.
Results
Serum Nitric Oxide
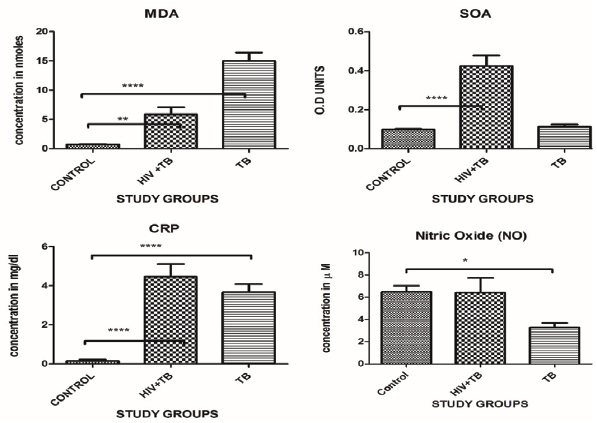
The serum NO showed a significant decrease in the values in TB study cohort 3.29±2.89 µM study cohort (p< 0.01) there was no significant increase in the serum nitric oxide in HIV-TB 6.40±8.99 µM study groups with respect to control group 6.46±3.75 µM [Table/Fig-1,2].
Means values of pro-oxidant markers in the study cohorts.
| Parameters | Mean±SE | Control (n=50) | Patients |
|---|
| HIV-TB n = 50 | TB n = 50 |
|---|
| NO | Mean (μΜ) | 6.46 | 6.40 | 3.29 |
| SEM (±) | 0.54 | 1.36 | 0.39 |
| TBARS | Mean (nmoles/ml) | 0.70 | 5.86 | 14.98 |
| SEM (±) | 0.06 | 1.16*** | 1.4**** |
| CRP | Mean (mg/dl) | 0.14 | 4.47 | 3.67 |
| SEM (±) | 0.06 | 0.63**** | 0.41**** |
| SOA | Mean (O.D Units) | 0.09 | 0.42 | 0.11 |
| SEM (±) | 0.003 | 0.05**** | 0.01 |
Significance is expressed in the form of p values.
*p < 0.01, **p < 0.001,
p < 0.0001,
p < 0.0001
NO - Nitric Oxide, TBARS - Thiobarbituric acid reactive species, CRP - C - reactive protein, SOA - Superoxide Anion
Showing pro-oxidant markers in the study groups
Legend: Significance is expressed in the form of p values. *p < 0.01, **p < 0.001
***p < 0.0001,****p<0.00001
Serum TBARS
The Thiobarbituric Acid Reactive Species (TBARS) were significantly increased in TB 14.98±10.86 nmoles/mL (p<0.001) and HIV-TB study cohort 5.86±7.88 nmoles/mL (p<0.001) with respect to control 0.70±0.45 nmoles/mL the p-value is significant [Table/Fig-1,2].
Serum CRP
The serums CRP levels were increase significantly in the both study groups TB (p<0.0001) and HIV-TB (p<0.0001) 3.67±3.03 mg/dL and 4.47±4.29 mg/dL with respect to control 0.14±0.47 mg/dL. Levels in the control group were in the normal range [Table/Fig-1,2].
Normal range as per the kit insert used was <0.6 mg/dL.
Superoxide Anion
The cellular Superoxide Anion (SOA) results showed a significant increase in the HIV-TB study group 0.42±0.36 OD Units (p<0.0001), while the results were not significance result in TB 0.11±0.10 OD units, study groups with respect to the control cohort 0.09±0.02 OD units.
Serum Catalase
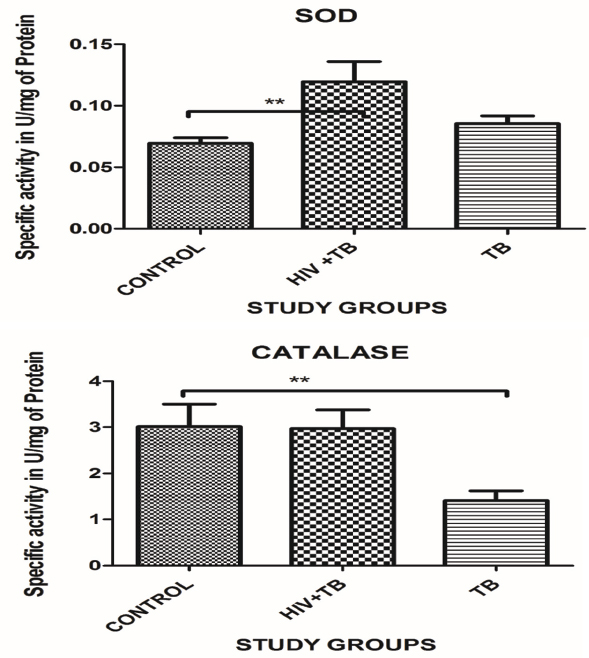
The serum catalase showed significant decrease with the TB study cohort 1.41±1.48 U/mg of protein, (p<0.01) and there was no significant change in the HIV-TB study cohort 2.97±2.74 specific activity in U/mg of protein with respect to the control cohort 3.01±3.46 U/mg of protein [Table/Fig-3,4].
Means values of anti-oxidant markers in the study cohorts.
| Parameters | Mean±SE | Control (n = 50) | Patients |
|---|
| HIV-TB n = 50 | TB n = 50 |
|---|
| CATALASE | Mean U/mg of Protein | 3.01 | 2.97 | 1.41 |
| SEM (±) | 0.48 | 0.40 | 0.20* |
| SOD | Mean U/mg of Protein | 0.06 | 0.11 | 0.08 |
| SEM (±) | 0.00 | 1.01** | 0.00 |
Significance is expressed in the form of p values.
p < 0.01,
p < 0.001,
***p < 0.0001, ****p < 0.0001, SOD - super oxide dismutase.
Showing anti-oxidant markers in all study groups.
Significance is expressed in the form of p values. *p < 0.01, **p < 0.001,
***p < 0.0001****p<0.00001
Serum SOD
The mean SOD levels were significantly increased in the HIV-TB study cohort 0.11±0.10 U/mg of protein (p<0.001) while there was no significant increase in the TB study cohort 0.08±0.04 as compared to the control study cohort 0.06±0.03 U/mg of protein [Table/Fig-3,4].
Total Serum Protein
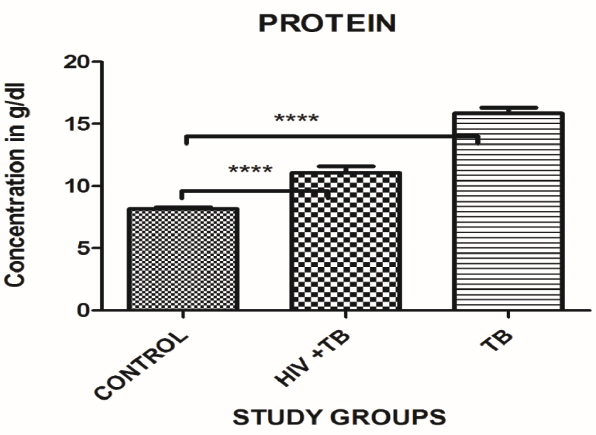
The total serum protein was assessed in the study cohorts. The total serum proteins showed a significant increase in TB study cohort (p<0.0001) 15.86±3.30 g/dL, while there was significant increase in the HIV-TB study cohort 11.07±3.68 g/dL (p<0.0001) with respect to control cohort 8.15±1.02 g/dL. Levels in the control group were in the normal range between 6.0-8.3 g/dL [Table/Fig-5,6].
Mean serum protein levels in the study cohorts.
| Parameters | Mean±SE | Control (n = 50) | Patients |
|---|
| HIV-TB n = 50 | TB n = 50 |
|---|
| Serum Protein | Mean (g/dL) | 8.15 | 11.07 | 15.868 |
| SEM (±) | 0.14 | 0.54*** | 0.44**** |
Significance is expressed in the form of p values. *p < 0.01, **p < 0.001,
p < 0.0001,
p < 0.0001
Mean serum protein levels in the study groups.
Significance is expressed in the form of p values. *p < 0.01, **p < 0.001,
***p < 0.0001, ****p<0.00001
Discussion
Mycobacteria are intracellular pathogens and replicate in the host macrophages. The host cells generate a huge amount of ROS to kill mycobacteria, which also contribute to inflammatory injury to the host cell. This ROS cause membrane LP leading to oxidative stress which can be assessed by measuring various LP markers in tissues and blood. These include MDA (measured as Thiobarbituric Acid Reactive Species-TBARS), Isoprostanes and 4-Hydroxy 2-Nonenal (4HNE) [24,25].
The present study shows the pro-oxidant and anti-oxidant levels in the study population. The serum NO levels were significantly decreased in TB study cohort and there was no significant change in the levels of NO in the HIV-TB co-infection study cohort. Serum levels of nitrite (NO2) and nitrate (NO3-) are used to estimate the level of NO formation, since NO is highly unstable and has a very short half-life [26]. Chronic inflammation enhances the production of NO, this can potentially mediate direct DNA damage. Alternatively, DNA damage via the generation of more persistent RNS may also be initiated [27]. iNOS and nitrotryramine reactive macrophages and Langerhans giant cells have been observed in certain granulomas. This suggests high NO production at these primary disease sites in Tuberculosis. All the patients were on anti-tubercular therapy as well as the anti-retroviral therapy. There could be the possibility of these drugs lowering down the levels of NO in the body. Lower NO levels in post-treatment may be due to a reduction in microbial load.
In this study we have found that the mean TBARS levels were significantly increased in the HIV-TB study cohort as well as the TB study cohort. Some studies have reported a considerable increase in the oxidative stress with increase in inflammation and with the severity of the diseases [28,29]. A significant increase in the lipid peroxidation in the form of MDA and conjugated dienes has been reported with some significantly marked clinical manifestations than in patients with small changes in X–Ray and sputum negative smear [30]. Some studies had reported that TBARS (MDA) levels increase in the pulmonary TB patients [3,31]. During the pulmonary inflammation, increased amounts of (ROS) and (RNS) are involved because of phagocyte respiratory burst. Thus, toxic free radical is implicated in the development of lung fibrosis, which may be a long-term effect of pulmonary tuberculosis. This also proves that there is a relation between the levels of LP measured as MDA (TBARS) and the severity of the disease. The earlier studies also shown the correlation between the elevated levels of MDA in pulmonary TB patients [32].
It is well established that tuberculosis co-infection with HIV increases replication of the virus and accentuates the progression towards AIDS, by enhancing the pro-inflammatory cytokines such as Tumour Necrosis Factor alpha (TNF-α) [29].
Enhanced synthesis of ROS further aggravates the condition.
HIV has been found to play a significant role in the augmentation of the intracellular SOA which is formed due to oxidative metabolism in cellular processes and vice versa [33]. The SOA has been implicated in the cell to cell transfer of HIV in cell cultures [34,35]. In the present study, the mean SOA levels in HIV-TB study cohort were significantly increased but there was no change in the TB study cohort. One study reported that at a higher concentration, (NO) combines with (SOA) to form peroxynitrite (ONOO) [36]. The synthesis of even a moderate flux of these molecules will result in the considerable oxidation and potential damage to the host cellular constituents, leading to the dysfunction of cellular processes, interruption of signalling pathways and induction of cell death through apoptosis and necrosis. Also, there could be a possibility of the generation of free radicals in the HIV patients along with TB infection, thereby increasing ROS formation more.
Serum CRP is used as a prognostic marker in many inflammatory disease conditions [37,38]. In the present study mean CRP levels were increased in the HIV-TB study cohorts as well as TB study cohort suggesting the potential role of CRP leading to the inflammation condition occurring in the co-infection study cohorts well as TB study cohort. Various studies have reported that higher CRP concentrations have been associated with the severity of TB and poor prognosis [39]. Patients with severe lung presentations, including cavitation, had significantly higher levels of CRP than patients without cavitation[40]. In vitro studies on mononuclear phagocytes have shown that M. tuberculosis and its components potentiates the release of Interleukin (IL) 6, which are regarded as inflammatory mediators. Many hepatic acute phase reactants including CRP are induced by IL-6) [41-43].
Various earlier studies have reported that HIV infected patients are in oxidative imbalance in the early stage of the disease [44,45].
To prevent any oxidative damage caused by the ROS production, there are some enzymatic anti-oxidants like SOD, Glutathione Peroxidase (GPx), catalase, Glutathione –S-Transferase (GST) which has a protective role.
Superoxide dismutase is zinc and magnesium containing enzyme is one of the antioxidant enzymes which protects against oxidants by converting superoxide radical to H2O2. The SOD activity was significantly increased in the HIV-TB study cohort, whereas the activity in the TB group was close to the activity seen in the control study cohort. An increase in activity of SOD is contradictory to most of the data available in this domain. This increase in SOD activity may however contribute to an increase in oxidative stress levels which in the event of an unchanged catalase activity in the HIV-TB group and significantly decreased activity in the TB study cohort will build up the hydroxyl radical ˙OH. Excessive ˙OH radical generation gives rise to the peroxidation by-products including MDA, 4-HNE etc. Thus, an increase in SOD activity and unchanged/decreased, catalase activity will be detrimental for maintaining the redox balance of the cell.
Catalase is an enzyme present in animal tissue, characterized by its power to convert H2O2 into water and oxygen. Catalase is constitutively expressed in type II pneumocytes along with SOD in the alveolar regions suggesting their protective role against the damage cause by free radicals in the present study we found that there was a significant decrease in the TB study cohort, while there was no change in the HIV-TB study cohort. This might suggest that the mycobacteria are resistant to H2O2 and organic peroxide. This may also indicate that the mycobacteria release catalase which can neutralize the H2O2 and lead to increased infectivity of mycobacteria in the immunocompromised patients [46,47].
The oxidative stress thus induced, has its effect on all macromolecular contents of the cell. One of the major effects of oxidative stress is on catabolism of proteins [48]. The catabolic effects of proteins lead to muscle wasting among other manifestation. In the present study, the significant hyperproteinemia was observed in TB and HIV-TB study cohort. Various studies have linked hyperproteinemia with conditions such as multiple myeloma [49] and other forms of cancer. The oxidative stress in the study patients may therefore have an impact on the protein catabolism leading to increased levels of proteins in the serum of the study cohort.
The balance between pro-oxidants and anti-oxidants in a cell is pivotal to maintaining the homeostasis of the cell. A tilt in this balance favouring the former hampers the physiological properties of the cells, leading to pathological outcomes. Tuberculosis, even after the known hundreds of years of its existence remains enigmatic. It affects millions of people globally causing severe morbidities and mortalities. The co-existence of the HIV-TB coinfection has only compounded the problems. The various biochemical markers although non-specific, may be used for effective management of patients with HIV, TB and HIV-TB co-infection.
Limitation
In the present study oxidative stress markers in HIV-TB co-infection were assessed at a single time point. A follow up study assessing the prognosis of the patients with respect to their redox markers will be of immense help in designing management strategies for the condition.
Conclusion
The present study showed that oxidative stress markers were significantly increased in the TB and HIV-TB coinfection population.
Since these factors contribute majorly too many of the co-morbidities in TB or HIV-TB individuals, modulating the redox balance in these individuals favouring a more anti-oxidant environment must be considered critically in the management of these conditions.