Placental trophoblastic cells possess the ability to proliferate, invade host tissue, avoid the host’s immune response and even metastasize. Gestational Trophoblastic Disease (GTD) is defined as heterogeneous group of interrelated lesions arising from the trophoblastic epithelium of the placenta after abnormal fertilization [1]. It includes various lesions such as pre-malignant lesions including hydatidiform mole (partial and complete type), while malignant lesions (gestational trophoblastic neoplasm) comprise invasive mole, choriocarcinoma, PSTT and epithelioid trophoblastic tumour [2].
Broad variations in the incidence of GTD have been reported in different parts of the world [3,4]. Risk factors include extreme of reproductive age, multiparity, past history of spontaneous abortions, endogenous oestrogens, high beta carotene diet, high animal fat diet, ethnicity, ABO blood group, environmental toxins, smoking, alcohol consumption, socioeconomic status, herbicide exposure etc., [1,4,5]. GTD lesions mimic growth pattern encountered in early normal placental development, non- molar abortions and a variety of non-trophoblastic lesions therefore histomorphological study is important to avoid confusion with their mimickers. The laboratory tests for serum hCG are most sensitive and specific for diagnosis of the trophoblast-related conditions, i.e., pregnancy and the GTD. It is noted that GTD produce hCG with a longer half-life, but an apparent half-life of more than three days suggests the presence of residual hCG-producing tumour tissue. In a treated GTD cases, a rise in hCG levels above the reference range suggests possible local or distant metastatic recurrence.
Thus, the present study on GTD includes clinicopathological evaluation with its clinical correlation.
Materials and Methods
The present descriptive, observational and analytical type of study was carried out at Krishna Hospital and Medical Research Centre, KIMS University, Karad, Maharashtra, India, in a tertiary care hospital. The study period was May 2012 to April 2016. All abnormal gestational related histopathological specimens from our hospital were included in the present study. The nongestational tissue was not included.
All clinically diagnosed, histopathologically confirmed cases were included. Detailed clinical, biochemical quantitative estimation of serum assay for beta hCG level (Bio. Meriex Pvt. Ltd. fluroscence method) and radiological (ultrasonography) assessment was done for diagnosis. All the specimens were routinely processed as per standard protocol to obtain tissue paraffin blocks; then sections were taken and stained by haematoxylin and eosin stain. Detailed microscopic evaluation was done and diagnosis was given as per WHO classification of GTD 2004. Diagnosis was then correlated clinically, radiologically and biochemically.
Statistical Analysis
All the data were analysed and studied by using software SPSS version 2.0. This study was approved by Institutional Ethical Committee.
Results
The total number of deliveries reported in the study period was 18,345, out of which a total of 77 cases of GTD were diagnosed. The prevalence of GTD in this tertiary care hospital was 4 per 1000 deliveries (0.4% or 1:238 deliveries). Over the study period, we received 986 samples of products of conception and three hysterectomy specimens out of which 77 (7.51%) were observed to be GTD cases. Out of which 74 (96.10%) were hydatidiform mole. There was a single (1.30%) case each of invasive mole, choriocarcinoma and PSTT [Table/Fig-1,2,3,4,5 and 6].
Specimen of partial mole.
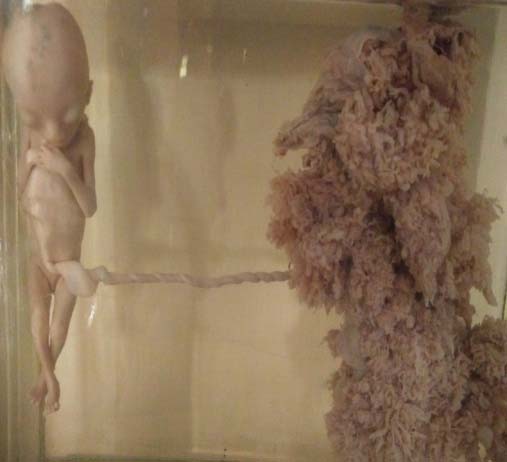
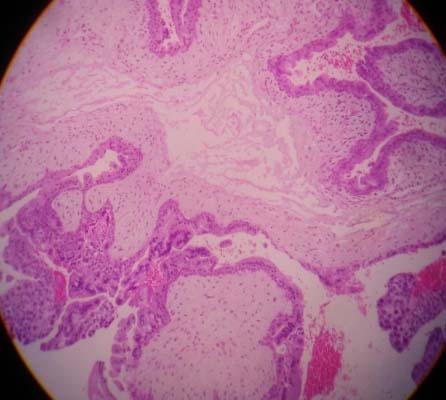
Photomicrograph showing a partial mole having villi with focal trophoblastic proliferation (H&E stained, 10X).
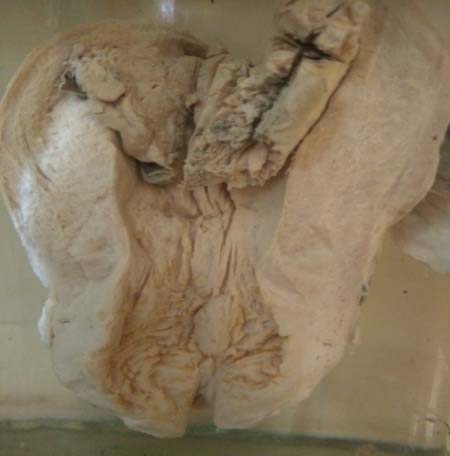
Specimen of complete mole showing grape like vesicle.

Photomicrograph showing complete mole having villi with circumferential proliferation of trophoblastic tissue. (H&E stained, 10X).
Specimen of choriocarcinoma.
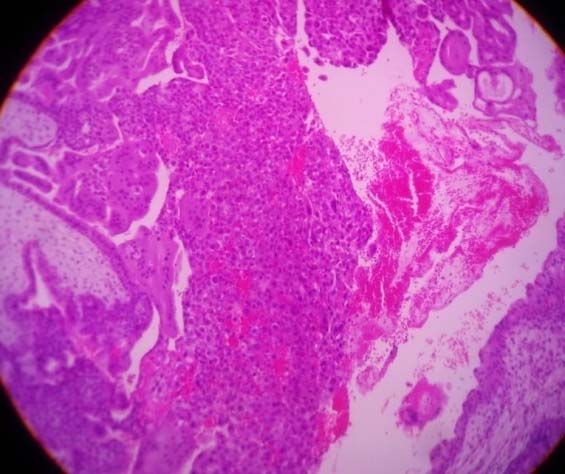
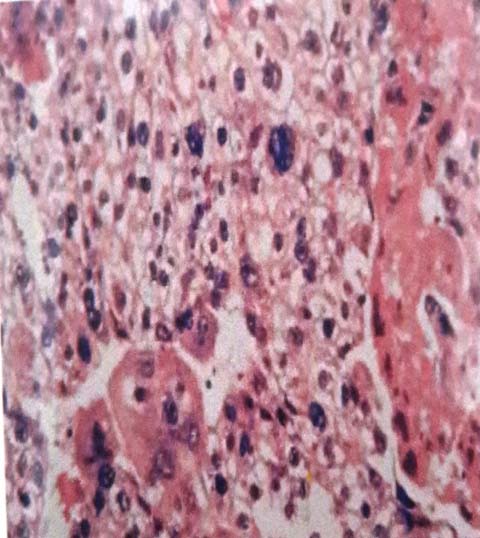
Photomicrograph showing malignant trophoblastic tumour – choriocarcinoma (H&E 40X).
Age-wise distribution of GTD has been given in [Table/Fig-7] and the distribution according to gestation has been given in [Table/Fig-8]. Bleeding per vagina was the most common clinical presentation. Details of other clinical presentations have been given in [Table/Fig-9]. Correlation of GTD with blood groups was evaluated and is given in [Table/Fig-10].
Various types of GTD according to age.
| Age group (Years) | Hydatidiform mole | Invasive mole | Chorio carcinoma | PSTT | Total | % |
|---|
| <20 | 03 | 00 | 00 | 00 | 03 | 3.90% |
| 20-25 | 43 | 00 | 01 | 00 | 44 | 57.14% |
| 25-30 | 24 | 01 | 00 | 00 | 25 | 32.46% |
| >30 | 04 | 00 | 00 | 01 | 05 | 6.50% |
| Total cases | 77 | 100% |
Distribution of GTD according to gestation.
| Trimester | Hydaditiform mole | Invasive mole | Chorio carcinoma | PSTT | No. of cases | % |
|---|
| Complete mole | Partial mole |
|---|
| I | 26 | 16 | 00 | 00 | 00 | 42 | 59.15% |
| II | 12 | 17 | 00 | 00 | 00 | 29 | 40.85% |
| III | 00 | 00 | 00 | 00 | 00 | 0 | 00.00% |
| Total | | | | | | 71 | 100% |
| Post gestation | 0 | 0 | 01 | 01 | 01 | 03 | 3.89% |
Various clinical presentations of GTD.
| Clinical presentation | Hydaditiform mole | Invasive mole | Chorio carcinoma | PSTT | Total | % |
|---|
| Bleeding per vagina | 70 | 01 | 01 | 01 | 73 | 94.80% |
| Amenorrhea | 71 | 00 | 00 | 00 | 71 | 92.20% |
| Pain | 46 | 00 | 01 | 01 | 48 | 62.34% |
| Hyperemesis gravidum | 06 | 01 | 01 | 00 | 08 | 10.39% |
| Passing grape like vesicles | 05 | 00 | 00 | 00 | 05 | 6.50% |
| Hyperthyroidism | 02 | 00 | 00 | 00 | 02 | 2.60% |
Table showing relation of different blood groups with GTD.
| Diagnosis | Blood group (ABO type) |
|---|
| A | B | AB | O |
|---|
| Complete mole | 29 | 2 | 2 | 12 |
| Partial mole | 8 | 3 | 6 | 12 |
| Invasive mole | 1 | 0 | 0 | 0 |
| PSTT | 0 | 0 | 0 | 1 |
| Choriocarcinoma | 0 | 0 | 0 | 1 |
| Total cases | 38 | 05 | 08 | 26 |
| Percentage | 49.35% | 6.50% | 10.40% | 33.75% |
In majority of cases beta hCG levels were between 50,000 to 100000 [Table/Fig-11]. The correlation between hCG level and GTD is given in [Table/Fig-12].
Distribution according to beta hCG level (pre evacuation).
| Beta hCG levels (mlU/mL) | No. of cases | % |
|---|
| 50,000 - <1,00,000 | 41 | 53.25% |
| 1,00,000 - <5,00,000 | 34 | 44.15% |
| 5,00,000 - <10,00,000 | 02 | 2.60% |
| Total | 77 | 100% |
| Mean ± SD | 267837.40mlU/mL± 696286mlU/mL |
Relation of beta hCG level and GTD.
| Beta hCG levels (mlU/mL) | Complete mole | Partial mole | Invasive mole | Chorio carcinoma | PSTT | No. of cases | % |
|---|
| 50,000 - <1,00,000 | 23 | 17 | 00 | 00 | 01 | 41 | 53.25% |
| 1,00,000 - <5,00,000 | 19 | 14 | 01 | 00 | 00 | 34 | 44.15% |
| 5,00,000 - <10,00,000 | 00 | 01 | 00 | 01 | 00 | 2 | 2.60% |
| Total | 77 | 100% |
Discussion
GTD consists of a spectrum of tumours and tumour like conditions characterized by proliferation of pregnancy associated trophoblastic tissue which may progress to malignancy [4]. There were wide geographical variations in the incidence of GTD as a result of differences in methodology, classification of mole, case detection and definition of the denominator. Incidence of GTD varies widely throughout the world. It was reported greatest in Asia, Africa, and Latin America and substantially lower in North America, Europe, and Australia [1,5,6]. The current study was an effort towards understanding GTD, its incidence, geographical variations, clinical presentation, investigations, histopathological examination, diagnosis, classification and its clinical correlation with GTD cases in a tertiary care hospital.
During the study period we observed a total of 77 cases of GTD. All the cases were uterine GTD. There was no case of extra-uterine gestational trophoblastic disease. The incidence of GTD in present study was 4/1000 deliveries (1 in 238 deliveries). A study done by Yakasai I et al., in 2015 at Nigeria, showed incidence of GTD was 4.5, Agrawal N et al., 4.17, Koirala A et al., 3.94 per 1000 deliveries and Sekharan P et al., showed high incidence rate i.e., five per 1000 deliveries [7-10]. These variations in the incidence of GTD are a result of differences in methodology, classification of mole, case detection. A study done by Shi YF et al., considered approximate incidence in Asia is about one in 250 deliveries [11], which showed concordance with present study.
The present study showed cases of GTD ranged from 19 to 38 years. GTD was most commonly noted in age group of 20-25 years with 44 cases (57.14%) followed by 25-30 years with 25 cases (32.47%) and least cases were found below 20 years only, 03 (3.90%) cases. Above 30 years, we noted 05 (6.49%) cases. Study by Taboo ZA had peak incidence of GTD was among 20-25 years age group [12]. The other study in India by Kumar N et al., had majority of GTD patients in age group of 20-25 years comprising 66% [13]. The mean age in our study of presentation was 24.5 years among all cases, which showed concordance with other studies by Agrawal N et al., and Mayun AA noted mean age of 23.9, 25.7 years [8,14]. In this region, early marriage may be related to early occurrence of GTD.
Our study showed 34 (44.15%) patients were primi gravida out of 77 cases of GTD, 22 (28.57 %) patients were second gravida whereas 15 (19.48%) were third gravida. The present study stated that GTD prevalence is more in primigravida women followed by second, third and next pregnancies with lower rates of GTD. Similar findings were noted in various studies by Brinton LA et al., Fatima M et al., and Saraf S [15-17]. Most of the cases presented in this study were in first trimester as seen in 42 (59.15%) cases and 29 (40.85%) cases were in second trimester. Another study by Taboo ZA had similar observation [12]. While study by Fatima M et al., observed in 31.6% cases in first trimester [18].
Usually patients clinically have a history of vaginal bleeding, often with symptoms of toxaemia. Frequently there is history of passage of grape like masses per vaginum [4]. In the present study most common presentation was bleeding per vagina with 73 (94.80%) cases, followed by amenorrhea with 71 (92.0%) cases. Similar results were in agreement with many other studies, like Taboo ZA, Berkowitz RS and Chhabra S et al., [12,18,19]. A study by Fatima M et al., noted similar results with 94.20% cases of bleeding per vagina [16]. In other Indian study, 97.78% cases presented as bleeding per vagina and 84.40% presented as amenorrhea [16].
In the present study, two patients came with complaints of Per Vaginal (PV) bleeding with sweating, palpitations and tachycardia. On further evaluation, these patients confirmed as having hyperthyroidism. These comprised of 2.60% of all GTD cases. The study done by Walkington L et al., reported 2% cases of hyperthyroidism [20]. Another study by Singh N et al., reported 2.20% cases having hyperthyroidism [21].
WHO prognostic scoring system for GTD has included ABO blood groups one of the prognostic factor. If female and male partner are with blood group either O or A, A or O; it carries better prognosis when compared with female having blood group B or AB [21]. Present study showed high incidence of GTD in a patients with blood group ‘A’ followed by blood group ‘O’ and least were noted in blood group ‘B’. Amongst them 64 were (83.11%) Rh positive. A study done by Parazzini F et al., found that ABO blood groups were associated with the risk of GTD [22]. Many studies stated that GTD was more prevalent with blood group A and similar results were encountered in our study [4,22].
Most of the GTD cases showed beta hCG levels between 50,000 – 1,00,000 mIU/ml. The lowest level of beta hCG of 65340 mIU/ml was observed in PSTT case. Not a single case was noted in below 50,000 mIU/ml or above range of 10,00,000 mIU/ml. Similar results were noted in other studies [11,23,24]. The serum beta hCG are most sensitive and specific for diagnosis of the trophoblast-related conditions, i.e., pregnancy and the GTD. It is important to regularly measure beta hCG levels in women diagnosed with complete or partial mole. An increasing level of total beta hCG is diagnostic of invasive disease and choriocarcinoma. It also helps to determine treatment response and recurrence of tumour.
Majority of GTD in our study were of hydatidiform mole among the 77 cases of GTD comprising 96.10%. Similar results were noted in many studies [6,16,25]. Complete mole is most common entity, comprising 57.34%. Partial mole was second most common entity observed in the present study comprising 1.33%. These results were in concordance with many studies [12,13,16]. On histopathology microscopically complete mole exhibits marked distended chorionic villi with cystern formation. The multifocal to circumferential proliferation of cytotrophoblast and syncytiotrophoblast is usually obvious [Table/Fig-10,11]. Fetal stromal blood vessels are absent. The distinguishing hydropic mole from early complete or partial mole is diagnostically challenging.
In the present study, we reported an unusual case of gravida 9 aged 30 with nine recurrent complete hydatidiform moles and no normal pregnancy comprising 1.3% among all hyadatidiform moles. Her blood group was B. Similar results were reported in a study by Belfort P and other researchers, showing 1.2% of recurrence among of all hydatidiform moles [26,27].
About 10% of patient with complete mole develops into invasive moles and 2.5% into choriocarcinoma. In the invasive mole, there is invasion of molar tissue into uterine wall which is source of haemorrhage. The lesion is benign but potential for haemorrhage and associated with persistent high level of hCG [17].
Choriocarcinoma is a highly malignant tumour of trophoblast. It can be gestational or non gestational [28]. About 50% of choriocarcinoma occurs following hydatidiform mole. Patient present with vaginal bleeding or rarely with distant metastasis. Diagnosis is confirmed by persistently raised hCG in blood and urine and on histopathology having malignant biphasic trophoblastic proliferation with extensive areas of haemorrhage and necrosis. Gestational choriocarcinoma responds very well to chemotherapy. Ancillary technique to assist histopathology diagnosis is available during recent years where various immunohistochemical markers and molecular techniques are used [29].
In the present study out of 77 cases, one case of choriocarcinoma was noted, constituting 1.30%. The patient was a 24 year, multipara who presented with amenorrhea and bleeding per vaginum. Her pre-treatment serum beta hCG level was 61,14,780 mIU/ml. Her blood group was O positive.
We reported a case of recurrent invasive mole in a 28 year, multipara. Her preoperative Beta hCG levels were 128000 mIU/ml. Her blood group was A positive.
PSTTs are very rare tumours. They represent a rare form of GTD. Out of these 77 GTD cases, 01 (1.30%) case of placental site trophoblastic tumour was observed in the present study a 30-year-old patient, G3L1A1, with a history of normal pregnancy followed by an abortion. Then she was presented with amenorrhea and bleeding per vagina. Her beta hCG levels before pre-evacuation was 65340 mIU/ml.
We reported a case of recurrent hydatidiform mole, an unusual case in a gravid 9 aged 30 with nine recurrent hydatidiform moles and no normal pregnancy. She had given history of similar episode of molar pregnancy in her mother. With a given familial history, this case was suggestive of Biparental Familial Complete Mole (BFCM). However, further investigation for the same were not done, hence, it cannot be confirmed as BFCM.
The standard treatment for women who may wish to have children in the future is to eliminate the mole by suction Dilation and Curettage (D&C). Women who no longer wish to have children may be able to have a hysterectomy and for tumours requires chemotherapy [30].
Limitation
The cytogenetic and molecular diagnosis in gestational disorders could not be done in our study due to the cost of investigations.
Conclusion
The prevalence of hydatidiform mole was higher among all entities of gestational trophoblastic disease. The complete hydatidiform mole was observed most common type in this study. The serum beta hCG levels are most sensitive and specific for diagnosis. Histopathological examination is helpful for confirmatory diagnosis. Follow up of such patients is essential for early detection of malignant trophoblastic tumours and to reduce mortality rate. Multi-centered studies are required in India to determine the true incidence and overall outcome of gestational trophoblastic diseases that will help in understanding the burden of disease and to produce the optimal outcome.