Pre-eclampsia-Eclampsia Syndrome (PES) has remained a major global public health threat in contributing significantly to maternal and perinatal morbidity and mortality. The global incidence of pre-eclampsia and eclampsia are 2.16% and 0.28%, respectively [1]. Corresponding figures in India are 1.97% and 0.43%, respectively [1]. Hypertension is the second most common direct cause of maternal mortality worldwide, accounting for 14% of all maternal deaths [2]. There is four and eight times higher risk for maternal death and Maternal Near Miss (MNM), respectively, in pre-eclampsia which exponentially increases to 42 and 60 times in eclampsia. PES is associated with several maternal complications like Disseminated Intravascular Coagulation (DIC), renal failure, Haemolysis, Elevated Liver Enzymes and Low Platelet (HELLP) syndrome, coma and pulmonary oedema. Risks of foetal death, early neonatal death, perinatal death, preterm birth and admission to Neonatal Intensive Care Unit (NICU) are also more frequent in pre-ecalmpsia (3 to 4 times) and eclampsia (4 to 8 times) compared to controls [1].
Pregnancy is a state of hypomagnesemia. The total and ionized serum magnesium (Mg2+) levels are not only significantly lower compared to non-pregnant women, but also tend to fall with advancing gestation, with further decrease in those who develop PES [3,4]. Mg2+ affects contractility and tone of blood vessels thereby regulating Blood Pressure (BP). So, it is obvious that Mg2+ depletion may be one of the aetiologies for PES.
Although the definitive treatment of pre-eclampsia and eclampsia is delivery, control of convulsions is the first and foremost principle of management of eclampsia. Magnesium sulphate (MgSO4) is the anticonvulsant of choice as it controls and prevents recurrence of eclamptic convulsions more effectively than diazepam and phenytoin, in addition to reducing feto-maternal morbidity and mortality [5,6]. The therapeutic serum Mg2+ concentrations advocated for effective control of eclamptic convulsions are 3.5-7 mEq/L (4.2-8.4 mg/dL) [7].
Among the two internationally recommended and widely used regimens - Pritchard’s and Zuspan’s regimens – the former is more popular in Resource-Poor Settings (RPS) [8]. After a loading dose, the drug is distributed throughout the body except a small amount which is left in the extracellular fluid. In thin women, the drug concentration is higher in serum with the maintenance regimen due to a lower total body volume. Unfortunately, use of this conventional supposedly ‘high dose’ Pritchard’s regimen, which is standardized for Western women, for management of PES in the lean and malnourished women of Low and Middle Income Countries (LMICs) showed multiple dose-related and potentially life-threatening toxicities like respiratory, renal and neuromuscular dysfunction, necessitating dose omission and resulting in low acceptance in RPS [9]. This stirred up a hunt for the most effective yet safest dose and regime of this revolutionary drug with a narrow therapeutic index. Since Asian women weigh approximately 2/3rd lesser than their US counterparts, reduction of dose for Asian women with lower BMI has been suggested in accordance with the observation that mean serum Mg2+ levels were significantly lower in women having a weight >70 kg than those with a body weight <70 kg [10]. Keeping in mind the small size of Asian women, the logistical and financial constraints pertaining to the availability of facilities for measurement of serum Mg2+ or availability of its antidote calcium gluconate, and paucity of trained staff for meticulous clinical monitoring of patients receiving MgSO4, several modified and alternate regimens have been described – lower loading and maintenance doses, single loading dose without maintenance or shorter maintenance course, etc., [9,11-15]. Although these Low Dose Regimes (LDRs) have shown promise in terms of efficacy, simplicity and safety [16-20], there are still limited studies with long term statistical data and lack of standardization of protocol thus hindering formulation of guidelines specific to Asian population.
The objective of the present study was to determine the minimum effective serum Mg2+ levels for the prevention and control of convulsions in Imminent Eclampsia (IE) and eclampsia using two regimens i.e., single loading low dose MgSO4 and standard Pritchard’s regimen. We also sought to evaluate and compare the efficacy, safety and the feto-maternal outcomes of these two regimens.
Materials and Methods
This prospective interventional comparative study was carried out from May 2010 to September 2011 in the Department of Obstetrics and Gynecology, Pt. JNM Medical College and Dr. BRAM Hospital, Raipur, Chhattisgarh, India, after approval from the Institutional Ethical Committee. All types of eclampsia (antepartum, intrapartum and postpartum) as well as those with Severe Pre-Ecalmspia (SPE) or IE for whom termination of pregnancy had been planned, were included, irrespective of their age, parity, Gestational Age (GA) or booking status. Those with doubtful history of convulsions, gestational hypertension, mild pre-eclapmsia, chronic hypertension, other causes of convulsions like epilepsy, Cerebrovascular Accidents (CVA), rupture of aneurysm, meningitis, encephalitis, cerebral tumours and metabolic abnormalities, those already treated outside with MgSO4 or other anti-convulsants before arrival at our hospital, those with contraindications to MgSO4 therapy (like myasthenia gravis, cardiac conduction defects, chronic renal disease, etc.,), those with complications at admission {like CVA, coma, renal failure, massive pulmonary oedema, aspiration pneumonitis, HELLP syndrome, abruption, DIC and shock (including sepsis)} and those with known medical disorders (like diabetes mellitus, heart disease, jaundice, blood dyscrasia, etc.,) were excluded.
Informed consent was obtained either from all subjects themselves, if conscious and well oriented, or from next of kin (usually the husband), if otherwise. Detailed history was elicited from participants or accompanying relatives, followed by thorough general, systemic and obstetric examination. BP was measured with standard sphygmomanometers, and systolic and diastolic end points were recorded as Korotkoff sounds 1 and 5, respectively. Injectable labetalol and/or oral nifedipine were administered in standard doses in hypertensive crisis (BP ≥ 160/110 mmHg). Baseline investigations like complete blood count including platelet count and peripheral smear, ABO and Rh, plasma glucose, renal and liver function tests, lactate dehydrogenase, coagulation profile, serum electrolytes, urine dipsticks for proteinuria and fundoscopy were performed for all. Eclampsia was managed as per standard principles. Antenatal corticosteroids were administered for foetal lung maturity in cases of anticipated preterm birth. All patients were monitored hourly for BP, Respiratory Rate (RR), oxygen saturation, Deep Tendon Reflexes (DTR) and urine output.
RR Cases were assigned into two groups: Study group received the single loading LDR and control group received the Pritchard’s regimen. In the study group, a single loading dose of 4 gm (20 ml of 20% solution) MgSO4 was given IV over not less than three minutes followed by 4 gm of 50% MgSO4 intramuscular (IM) in each buttock for control of convulsions in eclampsia. No maintenance dose was given. Prophylactic dose for SPE and IE was 8 gm of 50% MgSO4 given IM (4 gm in each buttock) without any IV or maintenance dose. This regimen was clearly described, duly structured and unanimously agreed upon and followed by all consultants and residents of the department to avoid errors or non-uniformity in its administration. The control group received the standard Pritchard’s regimen - loading dose of 4 gm MgSO4 (20 ml of 20%) IV over not less than three minutes which was immediately followed by 10 gm IM (20 ml of 50%, 5 gm in each buttock) in those with eclampsia. Maintenance dose of 5 gm (10 ml of 50%) MgSO4 was given 4 hourly IM in alternate buttocks after assuring presence of DTR, RR ≥ 12 breaths per minute and urine output of at least 100 ml in last four hours. In those with SPE and IE, 10 gm of 50% MgSO4 was administered IM (5 gm in each buttock) followed by 5 gm IM 4th hourly in alternate buttocks as prophylaxis for convulsions. The regimen was continued till 24 hours after delivery or after last convulsion, whichever was later.
In case of recurrence, defined as occurrence of convulsion after 30 minutes of loading dose in either regimen, additional 2 gm MgSO4 (10 ml of 20%) was administered IV over two minutes. If there was recurrence even after administering such additional doses twice, it was considered as failure of regimen and MgSO4 was discontinued. Instead, an infusion of 1000 mg of phenytoin was started after diluting it with 200 ml of normal saline and given over a period of one hour followed by phenytoin 100 mg IV 8th hourly. In both groups, serum Mg2+ levels were assessed before start of therapy, and at 30 minutes and four hours after start of therapy. Levels were also estimated before administering repeat doses in those with recurrent convulsions and in those with clinical manifestations of hypermagnesemia (loss of DTR, muscular paralysis, respiratory depression, cardiac arrest). In case of toxicity, further doses were withheld, 1 gm (10 ml) of 10% calcium gluconate was administered IV, slowly over 10 minutes, and ventilator support was provided, if needed.
Obstetric management was carried out after maternal stabilization. Patients with unfavorable cervix but adequate pelvis underwent induction of labour with dinoprostone or misoprostol. Those admitted in labour were augmented with oxytocin and amniotomy as appropriate, and allowed Vaginal Delivery (VD) under partograph monitoring. Caesarean Section (CS) was performed wherever indicated. Active management of third stage of labour was done routinely for all. Babies, who were born preterm, asphyxiated or with low birth weight, were managed in NICU. Maternal and neonatal morbidity were monitored till discharge and any maternal and/or perinatal mortality was noted.
The primary outcome measures were efficacy and safety of both regimens. Efficacy was determined by the minimum effective serum Mg2+ levels required for prevention and treatment of convulsions, and the rate of development or recurrence of convulsions in SPE and eclampsia, respectively. Safety was assessed by the frequency of adverse effects of MgSO4, need to discontinue or defer MgSO4 in view of clinical and/or biochemical hypermagnesemia and need to administer calcium gluconate. The secondary outcome measures included maternal morbidity (DIC, renal failure, intracranial haemorrhage, coma, HELLP syndrome and pulmonary oedema), maternal and perinatal mortality, and obstetric outcomes (mode of delivery, birth weight, Apgar at 5 minutes). Perinatal and obstetric outcomes were estimated only for those who delivered at our hospital.
Statistical Analysis
Continuous variables were expressed as mean±standard deviation whereas categorical parameters were expressed as percentages. The unpaired t-test and Chi-square test were used to compare the continuous and categorical variables, respectively. A p-value ≤ 0.05 was taken as a level of statistical significance. Data were analysed using SPSS PC version 22.0 (IBM Corp., Armonk, NY, USA)
Results
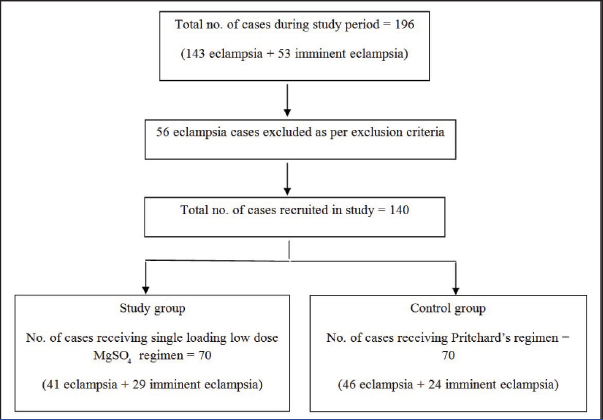
The study protocol and allocation of cases is illustrated in [Table/Fig-1]. A total of 87 cases of eclampsia, including 10 postpartum women, and 53 cases of IE were recruited into the study. Both the groups included a total of 70 patients each.
Flow diagram of study protocol.
[Table/Fig-2] depicts the demographic characteristics of both groups. Both groups were similar in terms of age, GA, parity and booking status. Majority of the patients in both groups were primigravida (65.7% overall) and unbooked (78.6% and 81.5% in study and control group, respectively), residing in rural areas with little or no access to health care.
Comparison of demographic parameters.
| Parameters | Total N (%) | Study group N (%) / mean± SD | Control group N (%) / mean± SD | p-value |
|---|
| Eclampsia | 87 (62.1) | 41 (58.6) | 46 (65.6) | 0.384* |
| Imminent eclampsia | 53 (37.9) | 29 (41.4) | 24 (34.3) | 0.384* |
| Booked | 28 (20) | 15 (21.4) | 13 (18.5) | 0.673* |
| Unbooked | 112 (80) | 55 (78.6) | 57 (81.5) | 0.673* |
| Rural | 102 (72.9) | 53 (75.7) | 49 (70) | 0.447* |
| Urban | 38 (27.1) | 17 (24.3) | 21 (30) | 0.447* |
| Maternal age (years) | - | 23.81 ± 4.95 | 23.7 ± 4.57 | 0.892† |
| Primigravida | 92 (65.7) | 45 (64.3) | 47 (67.1) | 0.722* |
| Mean GA (weeks) | - | 34.27 ± 4.06 | 33.1 ± 4.8 | 0.122† |
GA = gestational age
Chi-square test used as statistical test for categorical variables
Unpaired t test used as statistical test for continuous variables
[Table/Fig-3,4] show the serum Mg2+ levels in both groups at different time points of therapy. At the start of therapy, majority of patients with eclampsia and IE had sub-therapeutic levels, with mean < 3 mg/dl in both groups. In eclamptic women, significantly higher proportion of subjects in the study group had serum Mg2+ levels < 4 mg/dl and higher proportion of women had levels > 4 mg/dl in the control group at 30 minutes and four hours of onset of therapy [Table/Fig-3]. The mean serum Mg2+ levels were significantly lower in the study group at 30 minutes as well as at four hours of therapy in both eclampsia and IE (p<0.001). Minimum effective levels for control of convulsions in eclampsia with the single loading LDR were 2.20 mg/dl and 1.7 mg/dl while corresponding levels for prophylaxis were 3.39 mg/dl and 2.1 mg/dl at 30 minutes and four hours of therapy, respectively.
Distribution of cases according to serum Mg2+ levels in eclamptic women.
| Serum Mg2+ (mg/dl) | Study group N (%) | Control group N (%) | p-value |
|---|
| At start of therapy |
| ≤3 | 32 (78.1) | 35 (76.1) | 0.828* |
| 3-4 | 8 (19.5) | 10 (21.7) | 0.798* |
| 4-5 | 1 (2.4) | 1 (2.2) | 0.934* |
| >5 | 0 | 0 | - |
| Mean ± SD | 2.53 ± 0.64 | 2.63 ± 0.5 | 0.417† |
| At 30 min after therapy |
| ≤3 | 14 (34.1) | 3 (6.5) | 0.001* |
| 3-4 | 22 (53.7) | 10 (21.7) | 0.002* |
| 4-5 | 5 (12.2) | 19 (41.3) | 0.002* |
| >5 | 0 | 14 (30.4) | 0.001* |
| Mean ± SD | 3.36 ± 0.62 | 4.57 ± 0.9 | <0.001† |
| At 4 hour after therapy |
| ≤3 | 18 (43.9) | 1 (2.2) | <0.001* |
| 3-4 | 20 (48.8) | 3 (6.5) | <0.001* |
| 4-5 | 3 (7.3) | 17 (37) | 0.001* |
| >5 | 0 | 25 (54.3) | <0.001* |
| Mean ± SD | 3.17 ± 0.62 | 5.06 ± 0.56 | <0.001† |
Chi-square test used as statistical test for categorical variables
Unpaired t test used as statistical test for continuous variables
Distribution of cases according to serum Mg2+ levels in imminent eclampsia.
| Serum Mg2+ (mg/dl) | Study group N (%) | Control group N (%) | p-value |
|---|
| At start of therapy |
| ≤3 | 26 (89.7) | 22 (91.7) | 0.803* |
| 3-4 | 0 | 2 (8.3) | 0.113* |
| 4-5 | 2 (6.9) | 0 | 0.19* |
| >5 | 1 (3.4) | 0 | 0.358* |
| Mean ± SD | 2.49 ± 0.75 | 2.45 ± 0.38 | 0.814† |
| At 30 min after therapy |
| ≤3 | 9 (31) | 0 | 0.003* |
| 3-4 | 16 (55.2) | 9 (37.5) | 0.2* |
| 4-5 | 4 (13.8) | 12 (50) | 0.004* |
| >5 | 0 | 3 (12.5) | 0.05* |
| Mean ± SD | 3.39 ± 0.56 | 4.37 ± 0.76 | <0.001† |
| At 4 hour after therapy |
| ≤3 | 13 (44.8) | 0 | 0.0002* |
| 3-4 | 13 (44.8) | 10 (41.6) | 0.817* |
| 4-5 | 2 (6.9) | 7 (29.2) | 0.032* |
| >5 | 1 (3.5) | 7 (29.2) | 0.009* |
| Mean ± SD | 3.16 ± 0.79 | 4.98 ± 0.52 | <0.001† |
Chi-square test used as statistical test for categorical variables
Unpaired t-test as statistical test for continuous variables
Both regimens were equally efficacious in control (97.6% vs 97.8%, p=0.934) and prevention of convulsions (96.6% vs 100%, p = 0.358), as evident from [Table/Fig-5]. Recurrence occurred only in one case in each group.
Comparison of efficacy of two regimens.
| Parameter | Study group N (%) | Control group N (%) | p-value* |
|---|
| Control of convulsion | 40/41 (97.6) | 45/46 (97.8) | 0.934 |
| Prevention of convulsion | 28/29 (96.6) | 24/24 (100) | 0.358 |
Chi-square test as statistical test for categorical variables
As far as feto-maternal outcomes are concerned, there was no statistical significant difference in the CS rate in both groups (36.9% vs 50.8%, p=0.112). While there was one maternal death in the control group, there was none in the study group. The patient who died remained undelivered. Maternal morbidity and perinatal outcomes were also comparable in both groups [Table/Fig-6].
Comparison of feto-maternal outcomes.
| Outcome | Total N (%) | Study group N (%) / mean ± SD | Control group N (%) / mean ± SD | p-value |
|---|
| Vaginal delivery* | 71 (54.6) | 41 (63.1) | 30 (46.2) | 0.053‖ |
| Cesarean Section* | 57 (43.8) | 24 (36.9) | 33 (50.8) | 0.112‖ |
| Forceps delivery* | 1 (0.8) | 0 | 1 (1.5) | 0.315‖ |
| Undelivered* | 1 (0.8) | 0 | 1 (1.5) | 0.315‖ |
| Maternal morbidity† | 56 (40) | 25 (35.7) | 31 (44.3) | 0.301‖ |
| Maternal mortality† | 1 (0.7) | 0 | 1 (1.4) | 0.316‖ |
| Live birth‡ | 101 (78.3) | 54 (83.1) | 47 (73.4) | 0.184‖ |
| Intrauterine foetal death‡ | 29 (22.5) | 11 (16.9) | 18 (28.1) | 0.128‖ |
| Early neonatal death§ | 13 (12.9) | 7 (13.0) | 6 (12.8) | 0.976‖ |
| Perinatal death‡ | 42 (32.6) | 18 (27.7) | 24 (37.5) | 0.235‖ |
| Mean birth weight (kg) | - | 2.18 ± 0.502 | 2.1 ± 0.65 | 0.435** |
| Mean Apgar at 5 mins | - | 7.17 ± 2.2 | 6.6 ± 2.8 | 0.201** |
Total N = 130 (10 postpartum cases excluded, 65 in each group)
Total N = 140 (70 in each group, including antepartum, intrapartum & postpartum cases)
Total N = 129 (65 in study group, 64 in control group)
Total N = 101 (54 in study group, 47 in control group)
Chi-square test used as statistical test for categorical variables
Unpaired t-test used as statistical test for continuous variables
Discussion
MgSO4 is recognized by the World Health Organization and the United Nations as both a priority medicine and a life-saving commodity for the treatment of SPE and/or eclampsia. Its superior efficacy compared to other anticonvulsants is well proved. The unanswered question is what should be an ideal, cost-effective as well as safe regimen and dosage well suited to the small built women in RPS. In India, Pritchard’s regimen has been modified at many places due to concerns over availability, safety, cost and religious monitoring [9,21-24]. Despite growing evidence in literature regarding the safety and efficacy of several LDRs, the available data are too limited to draw reliable conclusions and hence it is not advisable to make any changes to the current treatment regimens [8,25]. Our study intended to compare the efficacy and safety of a ‘single loading abbreviated body friendly’ LDR with the standard Pritchard’s regimen.
PES is more common in the first pregnancy and in women < 25 years of age [14,18,19,23,24]. Findings of our study corroborate these facts. In most studies, as ours, patients presented preterm with a GA ranging between 32-36 weeks [9,13,24]. In others, majority presented at term [15,22,23]. Most studies, including ours, have observed a high frequency of unbooked cases from low socio-economic strata residing in rural areas with low BMI thus undermining the importance of adequate antenatal care for good pregnancy outcomes [14,22]. MgSO4 toxicity is dose related; administering the Pritchard’s regimen in these small built women may therefore be hazardous.
We found that at the beginning of MgSO4 therapy, majority of patients with eclampsia and IE in both the groups had hypomagnesemia, in accordance with others’ observations [3,4]. In our study, while the Pritchard’s regimen achieved therapeutic levels within 30 minutes of therapy, the mean Mg2+ levels remained lower than the recommended therapeutic range at 30 minutes and four hours of therapy in the single loading LDR. Our findings are similar to those of some authors who had found subtherapeutic levels with their LDRs [11,15] but in contrast to few others [17]. These variations could be due to different pharmacokinetics of the drug administered in different doses, routes and protocols in different studies. Nevertheless, the efficacy of our regimen is comparable to the conventional as well as other LDRs.
Several LDRs tried in several LMICs have demonstrated an efficacy of > 90% in the control and prevention of eclamptic convulsions, which is comparable to that of the Pritchard’s regimen [9,11-24]. Noteworthy in the present study is that, despite subtherapeutic levels, the single loading LDR could effectively control eclamptic convulsions in 97.6% cases, demonstrating similar efficacy as that of the Pritchard’s regimen (97.8%, p = 0.934). The recurrent convulsion rate of 2.4% observed in our study is similar to few studies [12] but remarkably lower than most other studies using various single loading dose regimens [18-20,23,24]. It is also much lower than that reported in studies evaluating Western women, possibly due to lower weight of our population [5,10]. BMI is an important factor determining the efficacy and toxicity of MgSO4 [26]. Begum MR et al., had suggested a small BMI to be the main reason for effectiveness of single loading LDR in women in developing countries [19]. Our study also demonstrated equally high efficacy of our LDR in prevention of convulsions in those with IE, compared to the Pritchard’s regimen (96.6% vs 100%, p= 0.358). This is in agreement with other studies using single loading dose regimens for eclampsia prophylaxis [13,20,24]. The total dose used in our regimen - 12 gm for eclampsia and 8 gm for IE – is 3 to 5 times less than that used in Pritchard’s regimen (39 gm in first 24 hours) with a dose reduction of approximately 70 to 80%. This suffices for our lean women, given its similar, if not superior efficacy, thereby making it a body-friendly regimen.
As regards safety, several authors have reported either no evidence of toxicity with LDRs [17,22,23] or lower toxicity than conventional regimens [9,15,24]. Our study too was devoid of MgSO4 toxicity in either regimen. The mean serum levels achieved with our LDR were much below the levels required to cause toxicity. This might encourage its use even with mild renal impairment which is frequently encountered in these women. Such a safe and effective regimen obviates the need for 1:1 monitoring, reduces overall cost, leads to more effective utilization of manpower and resources due to involvement of relatively less number of health care professionals for patient care and better patient satisfaction due to quicker return to postnatal wards and shorter hospital stay, thus making it an attractive option in RPS. Also, single administration eliminates risk of tissue necrosis and abscess formation associated with repeated painful IM injections thus improving patient compliance. It would especially be a boon in community settings where a single loading dose before referral would ensure safe seizure-free transit to higher centres.
The overall CS rate was high in our study (43.8%). Furthermore, the CS rate in our LDR group (~37%) was quite higher than most other reports [12-14,21,22]. It is well known that the definitive therapy in eclampsia and IE is termination of pregnancy and seizure-to-delivery interval has to be minimized. Our institute, being a tertiary care referral centre, received most cases that were either remote from term, not in labour, with unfavourable cervix or with additional obstetric risk factors thus favouring CS. Such high CS rates (>30%) have been noted in few other studies as well [9,11,18,24]. However, the CS rates were similar in both regimens in the present study. This implies that the LDR does not appear to alter labour outcomes or increase likelihood of CS compared to the Pritchard’s regimen. This agrees with certain studies [13,15,17].
Another remarkable finding of our study is zero maternal mortality with the use of single loading LDR which is supported by several authors [9,11,13,15]. Although some researchers have reported maternal deaths with various LDRs [14,19,21,22], the mortality rates are similar to those of conventional regimens [17,18,20,24]. Moreover, most of these deaths have occurred due to complications of eclampsia or other high risk obstetric conditions rather than due to MgSO4 overdose.
LDRs are associated with comparable maternal morbidity as in standard regimens [18,24]. Our study too affirms this. The incidence of maternal complications in our study is higher than most reports [9,23,24], explicable by the high turnover of referred unbooked cases with multiple high risk factors presenting late to our tertiary care hospital.
There were no differences in any of the perinatal outcomes between the two groups thus emphasizing that the low dose regimen is not inferior to the standard regimen in preventing adverse perinatal outcomes, as depicted in other studies too [13,16,18,24]. As expected, due to prematurity, mean birth weight was < 2.5 kg and mean Apgar at 5 mins was around seven in both groups. The perinatal mortality in our LDR was 27.7% which is comparable to several studies [12,21-23].
Limitation
Firstly, the number of cases recruited in our study was less as those with complications at admission or those who had already received MgSO4 outside were excluded. Secondly, it was a non-randomized comparative study. Thirdly, we did not do an economic analysis of the two regimens.
Conclusion
Single loading LDR is an equally efficacious, safer, cheaper and more body-friendly alternative to the Pritchard’s regimen for both prophylaxis and therapy of eclamptic fits even at subtherapeutic serum concentrations with similar feto-maternal outcomes. In developing countries where the maternal morbidity and mortality due to pre-eclampsia-eclampsia is relatively higher, resorting to such shorter low dose courses of MgSO4 therapy with minimal monitoring would be a welcome sea-change in the peripheral health centres with limited resources and high patient turnover. However, our results need validation from adequately powered, well designed large scale randomized trials before a change from standard Pritchard’s regimen to single loading LDR can be recommended, tailored to the Indian women with lower BMI.
GA = gestational age
*Chi-square test used as statistical test for categorical variables
†Unpaired t test used as statistical test for continuous variables
*Chi-square test used as statistical test for categorical variables
†Unpaired t test used as statistical test for continuous variables
*Chi-square test used as statistical test for categorical variables
†Unpaired t-test as statistical test for continuous variables
*Chi-square test as statistical test for categorical variables
*Total N = 130 (10 postpartum cases excluded, 65 in each group)
†Total N = 140 (70 in each group, including antepartum, intrapartum & postpartum cases)
‡Total N = 129 (65 in study group, 64 in control group)
§Total N = 101 (54 in study group, 47 in control group)
‖Chi-square test used as statistical test for categorical variables
**Unpaired t-test used as statistical test for continuous variables