The goal of periodontal therapy is the regeneration and reconstruction of the lost supporting hard and soft tissues of the tooth to improve its ability in load transfer to the bone. One of the major factors inhibiting predictable periodontal tissue regeneration appears to be the nature of the diseased root surface [1]. The root surface associated with periodontitis undergoes considerable alterations resulting in the destruction of the fiber attachment system. Root surface debridement of the teeth produces a smear layer that contains microorganisms and toxins which could interfere with periodontal healing [2,3]. Root biomodification agents are often used to remove the smear layer and to expose collagen fibers making the root surfaces biologically acceptable which in turn is necessary for the success of regenerative procedures. Studies have shown that citric acid and EDTA can cause collagen fiber exposure which augments the connective tissue attachment to the root surface [4]. Further, the blood element linkage to demineralised roots and clot stabilization by collagen fibres are vital for the wound healing and success of periodontal surgery. The aim of this in vitro study was to evaluate the distribution of fibrin network linkage to dentin surfaces which were conditioned with citric acid and EDTA. The hypothesis of the study is that the application of root biomodifiers like citric acid and EDTA would enhance the fibrin network linkage to the dentin surface thereby favorably promoting periodontal wound healing.
Materials and Methods
The current study was designed to be an in vitro study. Patients attending the Outpatient Department of Periodontics, Tamil Nadu Government Dental College and Hospital, Chennai, India, were screened for the study. Ten chronic periodontitis patients without any systemic complications were recruited. Sample size was taken based on the article published by Fábio Renato [5]. CRIS guidelines for in vitro studies state that most published in vitro studies do not include calculation of sample size but instead a non-parametric test can be chosen to analyse the data set [6]. The nature of the study was explained to the patients including extraction of teeth with hopeless prognosis and written consent for the study was obtained. Teeth affected by chronic periodontitis exhibiting Grade III mobility and indicated for extraction were selected for the study.
Sample Preparation
A total of ten freshly extracted teeth were collected and washed in normal saline to remove the blood. The soft tissue and other debris found on the root surfaces were removed with curettes. The teeth were stored in distilled water to avoid dehydration until ready for processing. The crown portion of the tooth at the cementoenamel junction and apical one-third of each root was cut and removed with diamond disc under copious distilled water coolant. The remaining midroot was sectioned longitudinally through the root canal with the diamond disc and the pulpal tissue was removed. Later, the root surface was flattened with high speed fissure bur to remove the cementum so as to expose the underlying dentin as described by Demirel K et al., [7], Frantz B and Polson A [8]. Three dentin blocks of approximately 4 mm x 3 mm x 1 mm in size were prepared from each root. One block each was allocated into the control Group A and the study Groups B and C. A total of 30 dentin blocks were thus prepared from 10 extracted teeth.
The control Group A specimens were treated with PBS (pH 7.4). The study Group B were treated with an aqueous solution of saturated citric acid (pH 1) (Sigma-Aldrich, Bengaluru, India). The study Group C specimens were treated with 24% EDTA gel (pH 7.4) (Prefgel;Biora,Sweden) with a cotton pellet. Each agent was applied on to the specimen for about three minutes with a cotton pellet which was changed every 30 seconds. The specimens were then subjected to five minutes rinse for three minutes with PBS and then allowed to air dry for 20 minutes. A drop of fresh human whole blood from a healthy volunteer was obtained and applied to the dentin blocks in all the three groups. The blood was then let to clot in a humidified chamber for about 20 minutes. The samples were further subjected to five minute rinses in PBS for three times.
Preparation of the Sample for SEM Analysis
Immediately after rinsing, the specimens were fixed with 2.5% glutaraldehyde (pH 7.2) at room temperature for 30 minutes. They were then washed thrice with PBS for five minutes each. The specimens were then dehydrated in a graded series of aqueous ethanol (25%, 50%, 70%, 90%, twice in 95%) for five minutes in each concentration. Finally, two dehydrations were performed using hexamethyl disilazine for three minutes each. The specimens were then air-dried and kept in glass vacuum desiccator.
The specimens were mounted in SEM stubs and sputter coated with a thin layer of a conductive material, gold palladium using a sputter coater (Polaron SC 500 sputter coater). They were then visualized using SEM [Stereoscan – 440] operated at an accelerated voltage of 15 – 20 KV.
Scanning Electron Microscope Analysis
The specimens were viewed at magnification values of x5000 at zero tilt angles. Each dentin block was scanned thoroughly to obtain an overview of the general surface topography. They were examined for the adsorption and adhesion of the blood element and the arrangement of the fibrin strands were also analyzed. The representative area in each dentin block which was characteristic of the general surface topography was selected at random and photomicrographed. In order to determine the degree of fibrin network linkage to the conditioned root surface, the following scores were used [5].
Score 0: Absence of fibrin network linkage to dentin surface
Score 1: Scarce fibrin network linkage to dentin surface
Score 2: Moderate fibrin network linkage to dentin surface
Score 3 : Dense fibrin network linkage to dentin surface
The SEM photomicrographs were evaluated and scored by a single examiner. The data collected was then subjected to statistical analysis.
Statistical Analysis
The statistical package SPSS PC version 17. 0 was used for statistical analysis. The normality test Kolmogorov-Smirnov and Shapiro Wilks test results showed that the distribution of fibrin network does not follow normal distribution. Therefore to analyse the data, Kruskal Wallis test is applied to compare the score values between the study groups. Pairwise comparison between the study groups was done using Mann Whitney test with Bonferroni correction. In the present study, p < 0.05 was considered as the level of significance.
Results
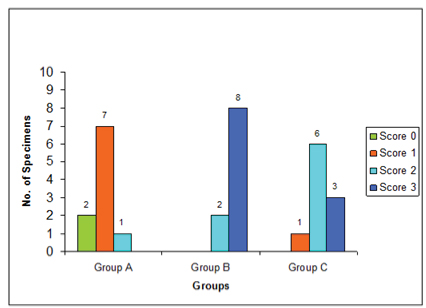
The characteristics of fibrin network formed on the dentin surfaces within each treatment groups seems to be quite consistent and there was little intragroup variations. The score distribution of the number of specimens in the control group and study Group B (citric acid) and C (EDTA) was given in [Table/Fig-1]. Under SEM, eight specimens in the citric acid group exhibited dense fibrin network linkage to the dentin surfaces [Table/Fig-2]. Six specimens in the EDTA group exhibited moderate fibrin network linkage [Table/Fig-3] and seven specimens in the PBS control group showed scarce fibrin network linkage to the dentin surfaces [Table/Fig-4].
Score distribution of the number of specimens in the control Group A and study Groups B and C.
Fibrin network formation on the dentin surface treated with citric acid.

Fibrin network formation on the dentin surface treated with ETDA.

Fibrin network formation on the dentin surface treated with PBS.
*PBS-Phosphate buffered saline.

For intergroup analysis, Kruskal Wallis test is applied to compare the score values [Table/Fig-5]. Further, Pairwise comparison between the study groups was done using Mann Whitney U test with Bonferroni correction.
Comparison of scores of fibrin network between the study groups using Kruskal-Wallis Test.
| Group | N | Mean ± Std. Dev | 95% CI for Mean | Mean rank | p-value |
|---|
| Lower | Upper |
|---|
| PBS | 10 | 0.90 ±0.568 | 0.49 | 1.31 | 6.35 | <0.001 (HS) |
| Citric acid | 10 | 2.80 ± 0.422 | 2.50 | 3.10 | 23.00 |
| EDTA | 10 | 2.20 ±0.632 | 1.75 | 2.65 | 17.15 |
| Total | 30 | 1.97 ±0.964 | 1.61 | 2.33 | |
*HS-Higher Significant, Std. Dev-Standard deviation, PBS-Phosphate buffered saline.
It was observed that the distribution of fibrin network is significantly greater in the citric acid and EDTA group compared to the control group PBS (p<0.001 and 0.012 respectively). However, there was no significant differences found when the two study groups, namely the citric acid and EDTA groups were compared (p =0.355) [Table/Fig-6]
Pairwise comparison between the study groups using Mann Whitney U test with Bonferroni correction.
| Group | p-Value |
|---|
| PBS vs Citric Acid | <0.001 (HS) |
| PBS vs EDTA | 0.012 (S) |
| Citric Acid vs EDTA | 0.355 (NS) |
*HS-Highly significant, NS-Not significant.
Discussion
The major goal of periodontal therapy is to restore and regenerate the periodontal attachment that has been destroyed by the disease process. The diseased root surface with destroyed fiber attachment greatly impair periodontal regeneration [9]. The diseased root surface harbour bacterial cells [10] as well as their cytotoxic products on the root surface. The elimination of microorganisms and their products from the root surface can be achieved by meticulous scaling and root surface debridement. However, this mechanical instrumentation results in a smear layer covering the root debrided surface [11,12]. The smear layer that is interposed between the root surface and adjacent connective tissue can retard the development of connective tissue attachment.
Conditioning of the root surface by topical application of root biomodifiers aid in the detoxification of remaining root surface contaminants and further dissolve the smear layer produced by root instrumentation [13]. In the present study, a saturated aqueous solution of citric acid and a chelating agent, EDTA has been used to condition the root surfaces. Citric acid and EDTA has been shown to remove the smear layer on the root surface, exposes the dentinal tubules and intertubular collagenous matrix, increases the wettability of the root surface resulting in the enhanced adhesion and adsorption of the blood elements to the root surface [14]. The exposed dentin or cementum collagen fibers on the root surface enhances migration, proliferation, adhesion as well as matrix synthesis of the cells involved in periodontal healing. Nevertheless, the collagen fibres enhances the stabilization of fibrin network of blood that is vital for periodontal tissue repair and regeneration [15-17]. This action further prevents the epithelial down growth, and forms a scaffold for cell development and mature collagen fiber attachment.
The results of the present study indicated that the distribution of fibrin network linkage to the dentin surface was significantly greater in the study groups namely the citric acid and EDTA groups. The dentin surface conditioned with citric acid and covered with human whole blood produced comparably dense fibrin network linkage. Boyko GA and Melcher AH in their in vitro study have shown that conditioning with citric acid causes partial demineralization of dentin which seems to improve mesenchymal cell adhesion possibly by a biochemical mechanism [18]. EDTA is less effective than citric acid and exhibited moderate fibrin network linkage when covered with human whole blood. EDTA exerts its demineralizing effect through chelating divalent cations at neutral pH [19,20]. However, no significant differences was observed in the distribution of fibrin network between the citric acid and EDTA group. Manzolli Leite FR et al., in their study have shown that conditioning with citric acid improves clot organization which supports the findings of this study [21].
The periodontal regeneration is essentially dependent on the adsorption and maturation of the fibrin clot that is positioned between the gingival flap and a periodontally compromised root surface [5]. Fibrin clot act as a critical determinants in regulating the early phases of wound healing. Blood elements formed onto the root surface during surgery and at wound closure must establish an attachment that endures normal physiologic as well as other potentially rupturing forces acting on the tooth – gingival flap interface. This attachment must remain stable during the early wound healing process so that the interface gets sufficient tensile strength on maturation to offset any impact from disruptive forces. The interaction among factors such as root surface, clot adhesion and connective tissue is necessary for a new connective tissue formation as opposed to a long junctional epithelium [5].
In that context, it is logical to suggest that in vitro protocols that maintain fibrin clot adhesion to dentin may favour wound maturation into a connective tissue attachment in vivo. However, protocols that are less successful in vitro should not be expected to support fibrin clot adhesion in a clinical scenario. Successful therapy depends on the uneventful maturation of a fibrin clot onto the root surface and to safeguard wound stability to take advantage of biologic agents applied onto the root surface with the intention to haste healing [13].
Limitation
Smaller sample size can be considered as a limitation for the present study. Furthermore, citric acid should be used with proper care as it has the potential to create an acidic environment in the surrounding tissues. In clinical perspective, the root biomodifiers can be used as an adjunct to conventional periodontal therapy as they modify periodontal wound healing favorably. However, further studies with larger sample size are required to confirm the effect of root biomodifiers on periodontal wound stability and healing.
Conclusion
From the findings of this study, it was observed that the dentin surface conditioned with citric acid and EDTA and covered with human whole blood produced almost comparable fibrin network linkage to dentin. Further, studies with large sample size are required to determine the effectiveness of root biomodifiers like citric acid, EDTA and to as certain their clinical implications in periodontal regenerative procedures.
*HS-Higher Significant, Std. Dev-Standard deviation, PBS-Phosphate buffered saline.
*HS-Highly significant, NS-Not significant.