Introduction
A large part of India’s population fulfils its healthcare needs from government healthcare delivery system such as Central Government Health Scheme, Armed Forces Medical Services, Employees State Insurance Corporation, State Medical Services etc. Use of medicines forms a large part of healthcare facility. For any condition, there may be numerous medicines existing, some of which probably got introduced more recently, may be more expensive and erroneously perceived to act better than the earlier known medications for the same ailment [1]. Even though more expensive, the newer medicine of the same drug class may not offer any distinct advantage in terms of the treatment outcomes. Also prescribers, many times choose to prescribe by the brand name whereas prescribing through the generic name may facilitate the dispensing as well as it turns out to be cheaper. What a physician prescribes to a patient for his/her ailment is largely a decision based on the patient’s clinical condition and physician’s personal choice for that condition based on his knowledge and experience. In free healthcare delivery systems, ideally a medicine that is not available in the medical store or dispensary should be avoided as far as possible by the treating physicians. Due to ready availability of large number of products and addition of many more almost every day to it, is impractical for any dispensary to stock all the possible options. Due to the large number of patients that the public healthcare system handles, there is a perpetual shortage of funds for procurement of medicines and other medical devices for patients [2,3]. In this situation of fund constraint, provisioning of medicines with an ever expanding list of ‘me too’ drugs, is an unmet challenge. Inadequate funding and inappropriate selection of medicines is one of the important reasons for non-availability of medicines in government hospitals [4]. Due to the reasons of non-availability, some of the prescribed medicines may have to be substituted on the spot with alternatives at the dispensary to avoid wastage of time and harassment to patients. Such substitution could fall into one of the two categories-generic or therapeutic. As per World Health Organisation (WHO) guidelines, generic substitution is defined as ‘the dispensing of a product that is generically equivalent to the prescribed product, with the same active ingredients in the same dosage form, and identical in strength, concentration and route of administration’. Therapeutic substitution or interchange is ‘the substitution of one medicine with another that differs in composition but is considered to have similar pharmacological activity (including side-effects) and therapeutic outcomes’ [5].
Generic Substitution: It involves substitution by a medicine with pure generic (e.g., Tablet paracetamol given instead of Tablet Crocin) or another brand (sometimes referred to as ‘branded generic’; e.g., crocin instead of calpol) with no change in dosage form. This is simple and easier to implement. Some more illustrative examples are elaborated in [Table/Fig-1]. The prescribed and the substituted drugs are considered equal as per regulatory norms and hence efficacy and safety of the generic alternative is ensured by law. There may also be resistance to generic substitution from the pharmaceutical industry or from the prescribing specialists who may consider the substituted product to be of inferior efficacy or quality. However, evidence suggests this approach to be valid with no compromise in safety and efficacy [6].
Illustrative examples of generic substitution.
| Brand prescribed | Manufacturer | Generic/branded generic dispensed | Manufacturer | Consequence on therapy |
|---|
| Tab C (Ranitidine 150 mg) | X Labs | Tab Ranitidine 150 mg | EN Drugs and Pharma | Clinically relevant differences unlikely as the substituted medicines are bioequivalent by law. |
| | | | |
| Tab E (Losartan 50 mg + Hydrochol-orothiazide 12.5 mg) | CS Ltd | Tab Losartan 50 mg + Hydrocho-lorothiazide 12.5 mg | Y Drugs and pharm-aceutics |
| Tab G(Sildenafil citrate 50 mg) | PNJ pharma Company | Tab P (Sildenafil citrate 50 mg) | IN and IN Ltd |
| Tab H(Omeprazole 20 mg + Domperi-done 10 mg) | UY Life Scineces | Tab R (Omeprazole 20 mg + Domper-idone10 mg) | X Labs |
| Inj F(Powder for Inj 1 g Amoxicillin with 0.2 g of Clavulanic acid) | MY pvt Ltd | Inj N (Powder for Inj 1 g Amoxicillin with 0.2 g of Clavulanic acid) | QP Ltd |
(Actual brand names and manufacturers have been replaced with fictitious names)
Limitations of Generic Substitution: Whenever we substitute a medicine, we provide something that was not intended by the treating physician. Still, generic substitution has greater acceptance from all concerned as all the medicines manufactured and marketed in India are bound by law to adhere to the pharmacopeia standards. Even though the medicine per se, also called Active Pharmaceutical Ingredient (API) remains same, different manufacturers are free to use other agents such as excipients (e.g., binders, stabilizing agents, preservatives) as per their choice to produce the formulation (tablet, capsule, injections etc.,). The regulations to market generics are based on bio-equivalence studies which are shorter in duration and less expensive in comparison to randomised clinical trials. The drug regulating agencies have issued elaborate guidelines and instituted stringent checks and balances while accepting bio-equivalence between two products [7,8]. Despite these safe guards, some patients may show significant differences (pharmacokinetic, pharmacodynamic or both) in responses to two medicines that are approved to be bioequivalent.
Another limitation of generic substitution is that the appearance of the substitute may be different. The differences in shape, size and colour of the dosage form may lead the patient to suspect erroneous prescribing and enhance possibility of non-compliance [Table/Fig-2].
a) Front view of tamsulosin 0.4 mg modified release tablet manufactured by company ABC; b) Front view of tamsulosin 0.4 mg modified release capsule manufactured by company XYZ; c) Rear view of tamsulosin 0.4 mg modified release tablet manufactured by company ABC; d) Rear view of tamsulosin 0.4 mg modified release capsule manufactured by company XYZ.
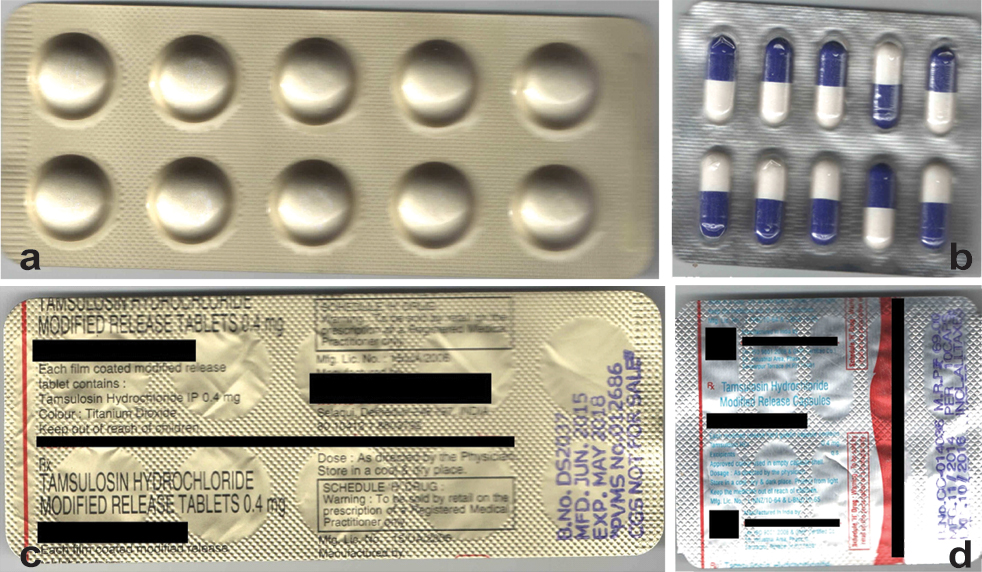
Considering the above limitations, this approach should be followed with caution and patients must be advised to seek medical attention sooner in case they sense something amiss about their medication whenever they have been dispensed a substitute.
Therapeutic substitution: All substitutions of medicine other than that described under generic substitution fall in this category. The substituted medicine may differ from the prescribed one on account of: (a) pharmacokinetics (e.g., metformin instead of metformin slow release); (b) an alternative molecule from the same pharmacological class (e.g., losartan instead of telmisartan); (c) an alternative molecule from a different pharmacological class (e.g., telmisartan instead of ramipril). Most accepted therapeutic substitutions are from the first and second categories [9]. The third category is usually undertaken with the approval of treating physician or by the treating physician directly. The essential assumption in therapeutic substitution is that the alternative provided is at least as effective as the prescribed one and has a comparable safety profile. Determination of equivalent effectiveness must be based on randomised clinical trials and not based only the mechanism of action. Sometimes, new drugs seek speedy market approval on the basis of comparison with placebo and not with an approved drug for the same indication. Evidence for non-inferiority (with respect to efficacy and adverse effect profile) or superiority amongst comparable medicines is determined by clinical trials and it is further strengthened by meta-analysis and more recently, network meta-analysis [10]. However, determining such evidence for comparison amongst medicines is laborious, expensive, time consuming and therefore exists for very few agents. As a result, many of the decisions taken during therapeutic substitution are based on presumptions. It may be worthwhile to take steps to either confirm or abandon these presumptions. A few examples of therapeutic substitution with their outcomes are mentioned in [Table/Fig-3] [11,17].
Illustrative examples of therapeutic substitution.
| Prescribed medicine | Manufacturer | Dispensed medicine | Manufacturer | Consequences on therapy | Reference Nos. |
|---|
| Tab AT 40 (Telmisartan 40 mg) once daily | ZX Pharma | Tab BT 50 (Losartan 50 mg)ORTab C 20 mg (Olmesartan) 20mg once daily | Y Drugs and PharmaceuticsORX Labs Ltd | Some short term studies indicate better control with telmisartan. | [11,12] |
| Tab CT 10 mg (Rosuvastatin) once daily | NY Biotech | Tab DT 20 mg (Atorvastatin 20 mg) once daily | Z Chemicals and Pharma | Rosuvastain is more potent but as efficacious other statins. | [13] |
| Tab ET 10 (Rabeprazole) 10 mg once daily | X Labs | Tab FT-10 (Omeprazole)10 mg once daily | EN Drugs and Pharma | Similar efficacy at lower cost with omeprazole. | [14] |
| Tab GT 5 (Prasugrel 5 mg) once daily | TD Labs | Tab HP (Clopidogrel 75 mg) once daily | Y Drugs and Pharmaceutics | Both are similar in efficacy and adverse effects. | [15] |
| JT FLEXPEN (Inj Insulin Detemir) | CS Ltd | KT AUTOPEN (Inj Glargine) | Y Drugs and Pharmaceutics | No clinically relevant difference in efficacy | [16] |
| Inj LT (Recombinant human erythropoietin alfa 2000 IU) | MY Pvt Ltd | Inj MT (Recombinant human erythropoietin alfa 2000 IU) | QP Ltd | Efficacy and safety profiles of biosimilar epoetin are similar to those of originator epoetin alfa | [17] |
(Actual brand names and manufacturers have been replaced with fictitious names)
Limitations of Therapeutic Substitution: All the issues mentioned for generic substitution are also equally applicable to therapeutic substitution. Additionally, there are some examples where different members of same pharmacologic class differ significantly in their biological effects and question the very concept of “class effect of medicines” [18]. Even for well established drug class such as beta blockers, there are differences in cardiovascular outcomes [19]. The significance of this difference and its effect on therapeutic outcome is open to speculation [20]. The impact of substitution on therapeutic outcome will also be affected by patient’s attitude, acceptance and understanding of the process. Studies have shown results that are both for and against therapeutic substitution [21,22]. If patients are not well explained about such a substitution they may wrongly carry an impression of inadequate/inappropriate treatment. Despite reasonable explanation, still few patients may demand the specific medicine or the specific brand and may remain dissatisfied if not accommodated. There may be adverse drug events with the substitute that possibly would not have been there if the originally decided medicine was dispensed [23]. Therefore, it is paramount, that patients consent for substitution must be verbally obtain and he or she be advised to concur with his physician regarding the same, though most of the physicians may stay opposed to medical substitution of all kinds considering infringement upon their rights to select appropriate medicines and also due to conflicts of interest with pharmaceutical industry.
Advantages of Therapeutic Substitution
1. Better therapeutic outcome: In our settings as would be in any public healthcare delivery system, the most common reason of substitution is non-availability of the prescribed agent. At the dispensary level, with the approval of a medical professional, though not necessarily of the one who originally prescribed the medicine, dispensing a substitute ensures that the patient gets treated for his condition with an appropriate drug. Due to various reasons especially in large busy hospitals, it is not possible for the prescribers in OPDs to be real time updated on the availability of a particular medicine. Also, it is impractical for the dispensary staff or the patients themselves to approach the prescribing physician every time with a request for change. Better therapeutic outcome and prevention of harm to patient is an associated advantage because therapeutic substitution also minimises therapeutic duplication [24]. Also, non-compliance due to non-availability of prescribed medicine is effectively prevented by therapeutic substitution and patient’s treatment is facilitated.
2. Better clientele satisfaction: The patient is more often satisfied and grateful if he is explained the reasons of non-availability of the prescribed medicine and substitute being provided, and also that the hospital is making all efforts to procure and provide the medicine that has been originally prescribed [25].
3. Saving on costs and time: Undoubtedly, generic substitution of branded medicines provides huge savings on expenditure without jeopardising the outcome. In addition, in institutional settings, if therapeutic substitution policies are implemented in systematic manner consulting all those concerned via., Drug and Therapeutic Committees, besides other general benefits of substitution policy, the inventories of medicines needed to be stocked can be reduced and procurement process also can be improved leading to more streamlined medicines supply, lesser stock outs, which may eventually reduce the need of substitution significantly [26]. At times, therapeutic substitutions may not be cheaper, but such an approach may be time saving.
Strategy for Medicines substitutions
Studies have revealed that analgesics, antibiotics, anti-hypertensives, anti-histaminics, proton pump inhibitors, benzodiazepines are some of the commonly substituted drug groups [27]. Also, it is wrong to believe that all medicines or medicine groups are amenable to substitution.
First and foremost, it has to be remembered that providing an alternative medicine through substitution of any kind must be done with the consent of the patient. It is broadly agreed that therapeutic substitution is best undertaken under hospital settings only. An unambiguous policy with the participation of all concerned needs to be formulated for implementing therapeutic substitution.
Once a therapeutic substitution is being considered, it is important to pay attention to equivalent dosages within the same pharmacological class and any special disability that the patient may be suffering from. For example, all the agents in the oral antidiabetic medicine group of ‘gliptins’ have same efficacy and need dose reduction in moderate to severe renal impairment, with the sole exception of linagliptin, which needs no dose reduction in such situation [28]. Therefore, in a patient with renal disease, linagliptin should not be substituted by any other gliptin for reasons of non-availability. This form of substitution, even though more challenging than generic substitution, is still manageable because, there are adequate scientific studies which have compared the medicines within the pharmacologic class and therefore comparative doses are proven.
The toughest call lies when therapeutic substitution across pharmacological classes is being considered. More often than not, the randomised clinical trials have rarely compared the original and the substituted medicine for efficacy and safety. There are many examples where the clinical outcome such as lowering of blood pressure with different drugs such as beta blockers and calcium channel blockers is same but the survival outcomes are not [29]. In such circumstances, the decision to substitute therefore should be left to the treating physician, if at all necessitated due to non-availability of the prescribed agent. There may be situations, however, when a pharmacist might not call or make the patient call his physician and coax or coerce the patient into buying a substitution considering the monetary gain. Such practice should be firmly discouraged.
The WHO recommends the formation of Drugs and Therapeutic committees in hospitals which will decide the hospital formulary and also approve substitutions. American College of Clinical Pharmacy in its policy statement on therapeutic substitution, issued in 2004, strongly recommended that there must be an agreement to study the outcome of therapeutic substitution. The outcomes should cover all aspects such as economic, clinical as well as humanistic. Humanistic outcomes may be the patient’s quality of life and satisfaction level with therapeutic substitution. Some well known institutions have their approved therapeutic substitution list in public domain [30,31].
‘Do Not Substitute’ or the Medicines that should remain exempt from substitution.
For reasons of patient safety, tolerability, efficacy and compliance, it is mandatory in case of some medicines to ensure that no medicine substitution of any kind takes place. Such clinical conditions and medicines are few in number.
As a rule of thumb, medicines with narrow therapeutic index should not be substituted at all. Some such examples are anti-epileptics, immunosuppresants, digoxin, and anticoagulants. In fact, there are numerous studies to support adherence to a particular brand, once the patient has got stabilised on it. Exceptionally, even a difference in excipient is known to cause havoc [32]. Therefore, medicines with narrow safety margins are recommended to be prescribed by brand names and even generic substitution is not permitted [33].
Biological medicinal products such as erythropoietin, growth hormones etc produced through genetic engineering should better be kept out of the scope of all types of substitution as bio-equivalence is more difficult to establish in such situations and patient’s immunological responses to the substituted drug may alter therapeutic outcomes [34].
In addition, any medicine that the prescriber specifically prohibits from substituting must not be substituted without at least a telephonic consult with him/her. Some of the justified reasons for prohibiting substitution of any kind could be to prevent harm to patient due to change in pharmacokinetics (extended release preparations versus regular ones), composition (fixed drug combinations) or familiarity with device (hormone pen injectors, rotahalers and other inhalation medication).
Legal status of dispensing medicine substitutes
In all countries where the state pays for the expenses of the medicines, it is quite acceptable practice to allow substitution of prescribed medicines to a greater or lesser extent. In fact in the early 20th century, there was a strict prohibition of substituting medicines in United States. However, things started changing in US and currently, therapeutic substitution is allowed and regulated in United Kingdom, many nations of European Union, Australia and New Zealand [35]. In the US, the process is formalised with the term ‘Therapeutic Interchange Program’ [27]. The practice of therapeutic substitution has gained acceptance over the years amongst prescribers, pharmacists and patients abroad. Most pharmaceutical associations in USA approve therapeutic substitution and so do physician associations. However, most disease specific associations in USA such as ‘Kidney foundation’ or ‘Heart foundation’ seem to oppose the concept.
In India presently, the governing document in the context of prescribing of all forms of medicines, The Drug and Cosmetics Act 1945 and rules as amended makes no mention of generic or therapeutic substitution [36]. The website of Pharmacy Council of India hosting the Pharmacy Practice Regulations, 2015 considers substitutions (of any kind) without the consent of Registered Medical Practitioner as malpractice and liable for disciplinary action [37]. Therefore, the substitution in India, howsoever necessary due to non-availability of the prescribed brand or medicine should be undertaken with the consent of the prescribing physician only. There is a renewed thrust in India for prescribing by generic names only [38].
The way ahead
For those practicing in institutions with attached dispensary viz., Government hospitals, prescribing through automated software linked to the dispensary is the best solution to avoid medicine substitution due to non-availability. The system can be programmed to allow prescribing of only those medicines that are available in the hospital dispensary and also inform about the alternative medicines in real time. Greater awareness amongst all stakeholders about therapeutic substitution will enhance the acceptance of the practice. Institutional implementation strategies will have to be formalised based on the WHO guidelines on drug and therapeutic committees. In studies carried out so far, the practice seems to be beneficial on the whole [39].
The drug regulatory bodies in all the countries across the world must take adequate steps to curb unregulated practices at the national level by making policies on therapeutic substitution that are more relevant and applicable to the local environment. An effort by regulatory bodies to ensure that similar drugs are also similar in appearance must be made. It must be ensured that drugs be dispensed by only qualified and legally authorized staff to avoid a havoc with patient life with wrong substitution especially with Look-Alike-Sound-Alike drugs.
Conclusion
Substitution of prescribed medicines at the dispensary level can never be unconditional. It may be hazardous unless utmost care is executed. Therapeutic substitution becomes necessary due to various reasons. It is best practiced in institutional settings under the overall supervision of a physician, with approved formularies and functioning Drug and Therapeutic committee. In most cases, substitution can be acceptable and beneficial provided all stakeholders in the matter consent to it. The national drug regulatory agencies need to come out with policies on therapeutic substitution tailor-made to healthcare delivery systems in that country.
(Actual brand names and manufacturers have been replaced with fictitious names)
(Actual brand names and manufacturers have been replaced with fictitious names)
[1]. Zetterqvist AV, Merlo J, Mulinari S, Complaints, complainants, and rulings regarding drug promotion in the United Kingdom and Sweden 2004–2012: A quantitative and qualitative study of pharmaceutical industry self-regulationPLoS Med 2015 12(2):e1001785 [Google Scholar]
[2]. Bajpai V, The challenges confronting public hospitals in india, their origins, and possible solutionsAdvances in Public Health 2014 [Google Scholar]
[3]. Reghu R, Vijayan M, Roshni PR, Procurement and distribution of medicines in government hospitals of Tamil Nadu - An overviewInternational Journal of Research in Pharmaceutical Sciences 2013 (4):96-100. [Google Scholar]
[4]. Wangu MM, Osuga BO, Availablity of essential medicines in public hospitals: A study of selected public hospitals in Nakuru County, KenyaAfrican Journal of Pharmacy and Pharmacology 2014 8(17):438-42. [Google Scholar]
[5]. Johnston A, Asmar R, Dahlof B, Hill K, Jones DA, Jordan J, Generic and therapeutic substitution: a viewpoint on achieving best practice in EuropeBr J Clin Pharmacol 2011 72:727-30. [Google Scholar]
[6]. O’Leary A, Usher C, Lynch M, Hall M, Hemeryk L, Spillane S, Generic medicines and generic substitution: contrasting perspectives of stakeholders in IrelandBMC Res Notes 2015 8(1):790 [Google Scholar]
[7]. Guidelines for Bioavailability and Bioequivalence Studies. Central Drugs Control Organisation, Ministry of Health and Family Affairs, Govt of India, New Delhi. [Cited in 2017]. Available from: http://cdsco.nic.in/html/be%20guidelines%20draft%20ver10%20march%2016,%2005.pdf [Google Scholar]
[8]. European Medicines Agency. Guideline on similar biological medicinal products. November 2004. [Cited in 2017]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf [Google Scholar]
[9]. Duerden MG, Hughes DA, Generic and therapeutic substitutions in the UK: are they a good thing?Br J Clin Pharmacol 2010 70(3):335-41. [Google Scholar]
[10]. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT, Evaluating the evidence from a network meta-analysisPLoS ONE 2014 9:e99682 [Google Scholar]
[11]. Samra SS, Dongre N, Ballary C, Desai A, Comparison of the efficacy, safety and tolerability of telmisartan with losartan in Indian patients with mild to moderate hypertension: a pilot studyJ Indian Med Assoc 2003 101(5):327-28. [Google Scholar]
[12]. Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J, Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertensionJ Clin Hypertens (Greenwich) 2001 3(5):283-91.:318 [Google Scholar]
[13]. Adams SP, Sekhon SS, Wright JM, Lipid-lowering efficacy of rosuvastatinCochrane Database Syst Rev 2014 11:CD010254 [Google Scholar]
[14]. Park JH, Park H, Lee DH, Sung IK, A randomized, double blinded, clinical trial to assess the efficacy and cost effectiveness of omeprazole compared to rabeprazole in the maintenance therapy of patients with gastroesophageal reflux diseaseJ Neurogastroenterol Motil 2013 19(2):219-26. [Google Scholar]
[15]. Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Prasugrel versus clopidogrel for acute coronary syndromes without revascularizationN Engl J Med 2012 367:1297-309. [Google Scholar]
[16]. Swinnen SG, Simon ACR, Holleman F, Hoekstra JB, DeVries JH, Insulin detemir versus insulin glargine for type 2 diabetes mellitusCochrane Database of Systematic Reviews 2011 7:CD006383 [Google Scholar]
[17]. Fishbane S, Shah HH, The emerging role of biosimilar epoetins in nephrology in the United StatesAm J Kidney Dis 2015 65(4):537-42. [Google Scholar]
[18]. Ono T, Sanai T, Miyahara Y, Noda R, Olmesartan is more effective than other angiotensin receptor antagonists in reducing proteinuria in patients with chronic kidney disease other than diabetic nephropathyCurr Ther Res Clin Exp 2013 74:62-67. [Google Scholar]
[19]. Mills EJ, Gardner D, Thorlund K, Briel M, Bryan S, Hutton B, A users’ guide to understanding therapeutic substitutionsJ Clin Epidemiol 2014 67:305-13. [Google Scholar]
[20]. Heffernan KS, Suryadevara R, Patvardhan EA, Mooney P, Karas RH, Kuvin JT, Effect of atenolol vs metoprolol succinate on vascular function in patients with hypertensionClin Cardiol 2011 (34):39-44. [Google Scholar]
[21]. Straka RJ, Keohane DJ, Liu LZ, Potential clinical and economic impact of switching branded medications to genericsAm J Ther 2015 (0):01-12. [Google Scholar]
[22]. American Heart Association. Drugs, therapeutic interchange and generic. [Cited in 2017]. Available from: https://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_435977.pdf [Google Scholar]
[23]. Strauss J, Greeff OBW, Excipient-related adverse drug Reactions: a clinical approachCurrent Allergy & Clinical Immunology 2015 28(1):24-28. [Google Scholar]
[24]. Hsu MH, Yeh YT, Chen CY, Liu CH, Liu CT, Online detection of potential duplicate medications and changes of physician behaviour for outpatients visiting multiple hospitals using national health insurance smart cards in TaiwanInt J Med Inform 2011 80:181-89. [Google Scholar]
[25]. Assefa F, Mosse A, Hailemichael Y, Assessment of clients’ satisfaction with health service deliveries at Jimma University Specialized HospitalEthiop J Health Sci 2011 21(2):101-09. [Google Scholar]
[26]. Kathleen H, Green T. Drug and therapeutic committees a practical guide. World Heath Organisation 2003. [Cited in 2017]. Available from: http://apps.who.int/medicinedocs/en/d/Js4882e/ [Google Scholar]
[27]. Gray T, Bertch K, Galt K, Gonyeau M, Karpiuk E, Oyen L, Guidelines for therapeutic interchange—2004Pharmacotherapy 2005 25:1666-80. [Google Scholar]
[28]. Gallwitz B, Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factorsTher Adv Endocrinol Metab 2013 4:95-105. [Google Scholar]
[29]. Thomopoulos C, Parati G, Zanchetti A, Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analysesJ Hypertens 2015 33(7):1321-41. [Google Scholar]
[30]. John Dempsey Hospital - Department Of Pharmacy Services. [Cited in 2017]. Available from: http://health.uconn.edu/pharmacy/staff-references/formulary-information/ [Google Scholar]
[31]. Therapeutic Interchange Program and Prescription Interpretations at Vancouver Community of Care. [Cited in 2017]. Available from: http://www.vhpharmsci.com/vhformulary/(2)%20VA%20Copy%20of%20BCHA%20Formulary%20THERAPEUTIC%20INTERCHANGE%20PROGRAM.pdf [Google Scholar]
[32]. Sharma HL, Sharma KK, Pharmacokinetics. In: Sharma HL, Sharma KK, AuthorsPrinciples of Pharmacology 2011 2nd ednHyderabad, IndiaParas Medical Publisher:25-55. [Google Scholar]
[33]. Talati R, Scholle JM, Phung OP, Baker EL, Baker WL, Ashaye A, Efficacy and safety of innovator versus generic drugs in patients with epilepsy: a systematic reviewPharmacotherapy 2012 32(4):314-22. [Google Scholar]
[34]. Macdougall IC, Casadevall N, Locatelli F, Combe C, London GM, Di Paolo S, Incidence of erythropoietin antibody-mediated pure red cell aplasia: the Prospective Immunogenicity Surveillance Registry (PRIMS)Nephrol Dial Transplant 2015 30(3):451-60. [Google Scholar]
[35]. Gumbs PD, Verschuren WM, Souverein PC, Mantel-Teeuwisse AK, de Wit GA, de Boer A, Society already achieves economic benefits from generic substitution but fails to do the same for therapeutic substitutionBr J Clin Pharmacol 2007 64:680e5 [Google Scholar]
[36]. Website of Central Drug Standards Control Organisation, Govt of India. [Cited in 2017]. Available from: http://www.cdsco.nic.in/forms/default.aspx [Google Scholar]
[37]. Website of Pharmacy Council of India. [Cited in 2017]. Available from: http://www.pci.nic.in/Circulars/Pharmacy%20Practice%20Regulations.pdf#page=1&zoom=auto,-104,297 [Google Scholar]
[38]. Website of Medical Council of India. [Cited in 2017]. Available from: http://www.mciindia.org/circulars/Public-Notice-Generic-Drugs-21.04.2017.pdf [Google Scholar]
[39]. Kairi JK, Implementing therapeutic substitution in a busy tertiary care hospital and reflections on dispensing challenges for a year long periodWorld Journal of Pharmaceutical and Life Sciences 2017 3(1):445-52. [Google Scholar]