Previous studies have explored the use of SGS and visual observation-based qualitative indices for evaluating parenchymal impairment in the salivary glands of patients who underwent 131I therapy for DTC [14-18]. However, clinicians disagree on how to consolidate and interpret SGS findings and symptoms, as the existing qualitative indices are not predictive of patients’ subjective symptoms of salivary gland dysfunction such as xerostomia. Further, these studies used relatively low cumulative doses (<10 GBq) of 131I and did not separately assess the relative function of the PG and SMG. Therefore, the purpose of this study was to develop an objective functional scoring system based on SGS findings that better correlates with and predicts the development of salivary gland dysfunction secondary to 131I therapy. Towards this end, we investigated multiple quantitative outcomes from the SGS of both the PG and SMG in a large population of patients with DTC after administering a varietyof different 131I doses (3.7–5.5 GBq).
Materials and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Kyushu University Hospital (Fukuoka, Japan) and written informed consent was obtained from all patients.
The data from 361 consecutive patients with DTC (96 men and 265 women) who underwent 131I therapy at Kyushu University Hospital at least once between January 2011 and December 2015 were retrospectively reviewed. Of the 361 patients, 314 patients (88 men and 226 women) who underwent SGS both before and after 131I therapy were analyzed in this study. All patients had undergone near-total or total thyroidectomy before 131I therapy and were histopathologically diagnosed with DTC. The inclusion criteria were as follows: (i) patients who underwent postoperative 131I radioablation for thyroid remnant tissue, or (ii) patients who underwent 131I therapy for metastatic or recurrent tumors from DTC. The exclusion criteria were as follows: (i) known history of salivary gland resection or external radiation therapy in the neck; (ii) baseline disease of salivary gland dysfunction before the initial 131I therapy (e.g., sialadenitis including Sjogren’s syndrome, sialolithiasis, or salivary gland tumor); (iii) usage of anticholonergic drugs and other drugs causing xerostomia; and (iv) suboptimal results or technical errors in SGS (e.g., incomplete immobility of the patients, inadequate injection technique). Consequently, 279 patients with DTC were included in this study. All 279 patients underwent SGS before initial 131I therapy and after one or more round(s) of 131I therapy. Additional 131I therapy was repeatedly performed as long as post treatment 131I scintigraphy shows 131I-avid uptake in residual tumors or metastasis based on American Thyroid Association (ATA) management guideline [19]. In these 279 patients, SGS was performed 12±1 months after the last 131I therapy. All patients underwent thyroid hormone withdrawal for at least three weeks before 131I therapy. A low-iodine diet was started two weeks before 131I therapy. The per treatment 131I dose ranged from 3.7 to 5.5 GBq. To prevent salivary gland damage, patients were instructed to suck on lemon candies during the first five days, beginning 1 day after 131I therapy, in accordance with a previous report [20]. The patients’ characteristics are shown in [Table/Fig-1].
Baseline characteristics of the patients.
| Variables | Pre treatment group (n=279) | Low dose group (n=193) | High dose group (n=86) | Symptom-negative group (n=236) | Symptom-positive group (n=43) |
|---|
| Age (years) | 16-75 (median:57) | 18-75 (median:58) | 16-73 (median:54) | 18-75 (median:57) | 16-75 (median:60) |
| Men/ Women | 78/201 | 48/145 | 30/56 | 71/165 | 7/36 |
| Papillary/ follicular/ papillary + follicular | 258/18/3 | 186/4/3 | 72/14/0 | 219/14/3 | 39/4/0 |
| TG level before 131I therapy (ng/ml) | 3.3-150000(median:22) | 3.3-7500 (median:11) | 6.9-150000 (median 155) | 3.3-150000 (median:17) | 5.0-31000 (median:105) |
| Cumulative 131I administered dose (GBq) | 3.7-33.9(median:4.5) | 3.7-9.5 (median:4.0) | 10-33.9 (median:13.6) | 3.7-33.9 (median: 7.4) | 7.6-33.7 (median:12.1) |
| TNM stage | | | | | |
| I | 51 (18%) | 38 (20%) | 13 (15%) | 48 (20%) | 3 (7%) |
| II | 21 (8%) | 5 (3%) | 16 (18%) | 13 (5%) | 8 (19%) |
| III | 30 (11%) | 26 (13%) | 4 (5%) | 27 (11%) | 3 (7%) |
| IV A | 101 (36%) | 84 (43%) | 17 (20%) | 92 (40%) | 9 (21%) |
| IV B | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IV C | 76 (27%) | 40 (21%) | 36 (42%) | 56 (24%) | 20 (46%) |
| ATA risk classification | | | | | |
| Low | 9 (3%) | 8 (4%) | 1 (1%) | 8 (3%) | 1 (2%) |
| Intermediate | 170 (61%) | 138 (72%) | 32 (37%) | 156 (66%) | 14 (33%) |
| High | 100 (36%) | 47 (24%) | 53 (62%) | 72 (31%) | 28 (65%) |
Salivary Gland Scintigraphy
Imaging was performed using a hybrid camera combining a dual-head γ-camera with a spiral computed tomography scanner within the same gantry (Infinia; GE Health Care, Milwaukee, WI), which was equipped with a low-energy parallel-hole collimator. The patient was in a supine position, and the camera was positioned for an anterior head-and-neck projection. Dynamic imaging was performed with a 64 × 64 pixel matrix at five minutes per frame starting immediately after a bolus intravenous injection of 370 MBq (10 mCi) 99mTc-pertechnetate. Imaging continued for 40 minutes after injection. At 23 minutes after injection, we administered lemon-juice stimulation to the mouth of each patient, without inducing movement, while imaging was continued.
Analysis of Salivary Gland Scintigraphy findings
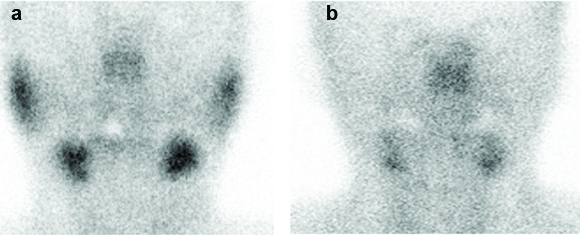
SGS imaging data were analyzed in several steps. First, saliva production was measured by assessing the degree of 99mTc-pertechnetate uptake in each salivary gland in the SGS images immediately before lemon-juice stimulation. A three-point uptake score was used to visually assess the degree of 99mTc-pertechnetate uptake in each salivary gland: 0–background; 1–decreased intensity; 2–normal intensity [Table/Fig-2]. Uptake scores of 0, 1, and 2 were respectively defined as intense dysfunction, no intense dysfunction, and no dysfunction in salivary glands, based on previous articles [15, 16]. The summed uptake score in the bilateral PG was defined as the PG uptake score and the summed uptake score in the bilateral SMG was defined as the SMG uptake score.
Pattern of the three-point uptake scores, as observed by salivary gland scintigraphy. a): The uptake score in the bilateral parotid and submandibular glands is 2. b): The uptake score in the bilateral parotid glands is 0 and that of the bilateral submandibular glands is 1.
Second, maximum secretion of saliva was measured as %WR. To do this, regions of interest were drawn on the dynamic images of the bilateral PG and SMG and time-activity curves were generated. The response of the salivary glands to lemon juice was noted on the time-activity curves as a sharp decline in the activity of the gland with a subsequent slow build up. Then, the %WR was calculated as follows [21]:
%WR = (cmax – post counts)/cmax×100
where “cmax” is the maximum activity counts before lemon juice stimulation and “post counts” minimum activity counts after stimulation [21]. Using this formula, the %WR in the PG (PG %WR) and the %WR in the SMG (SMG %WR) were respectively calculated. Decreased %WR was defined as a decrease in %WR more than 10% between before the initial therapy after the last therapy, according to the literature [15].
Third, the %WR was also assessed with a three-point %WR score based on the findings in the patients before initial 131I therapy, as follows: 0, decreased %WR = 100%; 1, 10% < decreased %WR < 100%; 2, %WR ≤ 10%. The %WR scores of 0, 1, and 2 were considered to indicate intense dysfunction, no intense dysfunction, and no dysfunction in salivary glands, respectively. The summed %WR score in the bilateral PG was defined as the PG %WR score and the summed %WR score in the bilateral SMG was defined as the SMG %WR score.
Finally, the total scores for the PG and SMG, including the uptake scores and %WR scores, were defined as the PG functional score and the SMG functional score, respectively.
Based on the administrated cumulative 131I dose, the 279 patients were categorized into the low-dose group (SGS following an131I therapy dose of<10 GBq, n = 193), and high-dose group (SGS following an 131I therapy dose of ≥10 GBq, n = 86). Additionally, the 279 patients were categorized into the, symptom-positive group (n = 43), and symptom-negative group (n = 236) based on the presence or absence of dry mouth symptom at the time of the SGS examination. The PG/SMG uptake scores, PG/SMG %WR scores, and PG/SMG functional scores were compared among pre treatment, low-dose, and high-dose groups, and among pre treatment, symptom-positive, and symptom-negative groups.
Analysis of association with dry mouth symptoms
Persistent subjective symptoms of dryness in the mouth and swallowing difficulty were judged as the presence of dry mouth symptom. The association between various clinical and radiological factors {age, sex, histological type, ATA risk classification, Thyroglobulin (TG) level before therapy (tumor marker), administered cumulative 131I dose, and PG/SMG functional scores} and dry mouth symptoms were analyzed. The predictive value for dry mouth symptoms was also evaluated. Age, sex, and histological type were evaluated at the time of histopathological diagnosis as DTC. ATA risk classification was determined according to the guideline [19]. TG level was measured immediately before an131I initial therapy under elevated thyroid-stimulating hormone levels.
Statistical Analysis
All statistical analyses were performed with the JMP® software package (version 8.0.2; SAS Institute, Cary, NC). Comparisons of the PG/SMG uptake score, PG/SMG %WR score, and PG/SMG functional score among pre treatment, low-dose, and high-dose groups and among pre treatment, symptom-positive, and symptom-negative groups were performed using Kruskal-Wallis tests and post-hoc pairwise tests. The correlations between clinical/radiological factors and dry mouth symptoms were analyzed by univariate and multivariate logistic regression analyses. Regression coefficients and odds ratios were calculated and 95% confidence intervals are given. The predictive value of dry mouth symptoms was determined using receiver operating characteristic curve analyses. Statistical significance for all tests was set at p< 0.05.
Results
Clinical outcomes
Dry mouth symptoms developed in 43 of the 279 (15.4%) patients with DTC after 131I therapy [Table/Fig-1]. Of these 43 patients, 10 were from the low-dose group (i.e., 5% of the low-dose group had dry mouth symptoms), and the other 33 were from the high-dose group (i.e., 38% of the high-dose group had dry mouth symptoms).
Comparisons among pre treatment, low-dose, and high-dose groups
The uptake scores, %WR scores, and functional scores in PG/SMG were significantly lower in the high-dose group than in the pre treatment and low-dose groups (p< 0.001 for all comparisons; [Table/Fig-3]). All three scores in PG were significantly lower in the low-dose group than in the pre treatment group (PG uptake score, p< 0.001; PG %WR score, p=0.002; PG functional score, p=0.001), although there were no significant differences between the pre treatment and low-dose groups in SMG [Table/Fig-3].
Differences in the SGS parameters among pre treatment, low-dose, and high-dose groups.
| Variables | Pre treatment group(n = 279) | Low-dose group(n = 193) | High-dose group(n = 86) |
|---|
| PG uptake score | 4.0 ± 0 | 3.1 ± 1.2* | 2.3± 1.5† |
| SMG uptake score | 4.0 ± 0 | 3.9 ± 0.4 | 3.2 ± 1.1† |
| PG %WR score | 4.0 ± 0 | 2.6 ± 1.5* | 1.9 ± 1.8† |
| SMG %WR score | 4.0 ± 0 | 3.9 ± 0.4 | 3.1 ± 1.2† |
| PG functional score | 8.0 ± 0 | 5.7 ± 2.6* | 4.2 ± 3.1† |
| SMG functional score | 8.0 ± 0 | 7.8 ± 0.8 | 6.3 ± 2.2† |
SGS = salivary gland scintigraphy, PG = parotid gland, SMG = submandibular gland, %WR = washout rate.
*p< 0.05 vs. pre treatment group, Kruskal-Wallis tests and post hoc pairwise tests;
†p < 0.05 vs. low-dose group, Kruskal-Wallis tests and post hoc pairwise tests.
Comparisons among pre treatment, symptom-negative, and symptom-positive groups
The uptake scores, %WR scores, and functional scores in PG/SMG were significantly lower in the symptom-positive group than in the pre treatment and symptom-negative groups (p< 0.001 for all comparisons; [Table/Fig-4]). All three scores in PG were significantly lower in the symptom-negative group than in the pre treatment group (p< 0.001 for all comparisons), although there were no significant differences between the pre treatment and symptom-negative groups in SMG [Table/Fig-4].
Differences in the SGS parameters among pre treatment, symptom-negative, and symptom-positive groups.
| Variables | Pre treatment group(n = 279) | Symptom-negative group(n = 236) | Symptom-positive group(n = 43) |
|---|
| PG uptake score | 4.0 ± 0 | 3.1 ± 1.2* | 1.2 ± 1.0† |
| SMG uptake score | 4.0 ± 0 | 4.0 ± 0.1 | 2.1 ± 0.9† |
| PG %WR score | 4.0 ± 0 | 2.8 ± 1.5* | 0.4 ± 0.9† |
| SMG %WR score | 4.0 ± 0 | 4.0 ± 0.3 | 2.0 ± 1.0† |
| PG functional score | 8.0 ± 0 | 5.9 ± 2.5* | 1.5 ± 1.6† |
| SMG functional score | 8.0 ± 0 | 7.9 ± 0.4 | 4.2 ± 1.7† |
SGS = salivary gland scintigraphy, PG = parotid gland, SMG = submandibular gland, %WR = washout rate.
*p< 0.05 vs. pre treatment group, Kruskal-Wallis tests and post hoc pairwise tests;
†p < 0.05 vs. low-dose group, Kruskal-Wallis tests and post hoc pairwise tests.
Relation factor analysis for dry mouth symptoms secondary to 131I therapy
Univariate logistic analyses showed that ATA risk classification, TG level before therapy, cumulative 131I dose, PG functional score, and SMG functional score were significantly associated with dry mouth symptoms. In the multivariate logistic analysis, only the PG functional score (χ2 = 11.8, odds ratio = 0.03) and SMG functional score (χ2 = 109, odds ratio = 0.0007) continued to show significant associations with dry mouth symptoms [Table/Fig-5].
Relation factor analyses for dry mouth symptoms.
| Characteristics | | Univariate logistic analysis | Multivariate logistic analysis |
|---|
| χ2 | Odds ratio | p | χ2 | Odds ratio | 95% CI | p |
|---|
| Age (years) | ≥45 vs. <45 | 0.01 | 0.96 | 0.92 | | | | |
| Sex | Women vs. men | 3.77 | 0.45 | 0.052 | | | | |
| Histological type | Papillary vs. follicular only | 0.22 | 0.76 | 0.64 | | | | |
| ATA risk classification | High vs. low/intermediate | 18.1 | 0.24 | <0.001 | 0.20 | 0.64 | 0.09–4.65 | 0.66 |
| TG level before therapy (ng/mL) | ≥230 vs. <230 | 12.0 | 0.28 | 0.0005 | 1.25 | 0.29 | 0.03–2.77 | 0.28 |
| Cumulative 131I dose (GBq) | ≥5.1 vs. <5.1 | 44.7 | 0.07 | <0.001 | 1.28 | 0.34 | 0.05–2.17 | 0.26 |
| SGS findings | | | | | | | | |
| PG functional score | ≤1 vs. >1 | 45.5 | 0.07 | <0.001 | 11.8 | 0.03 | 0.003–0.32 | 0.0006 |
| SMG functional score | ≤6 vs. >6 | 176 | 0.002 | <0.001 | 109 | 0.0007 | 0.0001–0.008 | <0.001 |
CI = confidence interval, ATA = American Thyroid Association, TG = thyroglobulin, SGS = salivary gland scintigraphy, PG = parotid gland, SMG = submandibular gland.
Predictive analysis for dry mouth symptoms
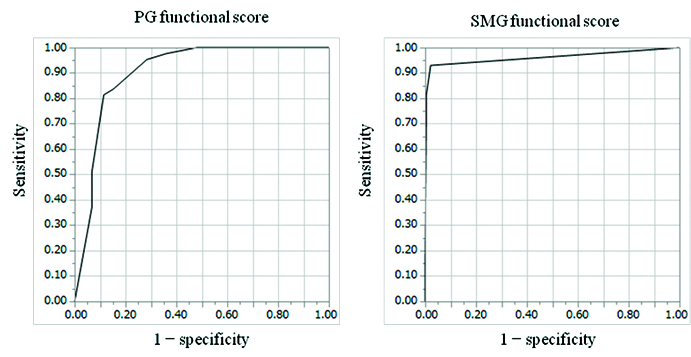
Results of the receiver operating characteristic analyses to determine the predictive value of the ATA risk classification, TG level before therapy, cumulative dose of 131I administered, PG functional score, and SMG functional score are shown in [Table/Fig-6]. Use of the optimal cut off thresholds for the SMG functional score differentiated the symptom-positive group from the symptom-negative group with the highest sensitivity, specificity, accuracy, and area under the curve [Table/Fig-6,7]. A significant difference was found between the area under the curve obtained from the PG and SMG functional score (p=0.04).
Predictive values of the risk factors for dry mouth symptoms.
| Risk factor | Sensitivity | Specificity | Accuracy | AUC |
|---|
| High-risk ATA group | 65% (28/43) | 69% (164/236) | 69% (192/279) | 0.67 |
| High pre treatment TG level (≥ 230 ng/mL) | 44% (19/43) | 83% (196/236) | 77% (215/279) | 0.66 |
| High cumulative 131I dose (≥ 5.1 GBq) | 77% (33/43) | 82% (193/236) | 81% (226/279) | 0.79 |
| Low PG functional score (≤ 1) | 81% (35/43) | 89% (209/236) | 87% (244/279) | 0.90 |
| Low SMG functional score (≤ 6) | 93% (40/43) | 98% (231/236) | 97% (271/279) | 0.96 |
Predictive value of the Parotid (PG) and Submandibular Gland (SMG) functional scores for dry mouth symptoms secondary to 131I therapy, as evaluated using receiver operating characteristic curve analyses. Receiver operating characteristic curves of the PG functional score (left) and SMG functional score (right) for predicting dry mouth symptoms are shown. The areas under the curve for the PG functional score and SMG functional score (0.90 and 0.96, respectively) were significantly different (p = 0.04).
Discussion
The aim in this study was to develop an objective functional scoring system based on SGS findings that correlates with and predicts the development of salivary gland dysfunction secondary to 131I therapy in DTC patients. We found significant differences in uptake, %WR, and functional scores in both PG and SMG among patients grouped by 131I dose or dry mouth symptoms. PG dysfunction appeared to occur before SMG dysfunction after 131I therapy. PG/SMG functional scores were shown to be independent risk factors for development of dry mouth symptoms.
In the present study, 15.4% of the patients with DTC (5% of the low-dose group and 38% of the high-dose group) developed dry mouth symptoms after 131I therapy. Previous studies have shown that the incidence of xerostomia due to salivary gland dysfunction after 131I therapy varies from 16 to 52% [10, 14-17], and this study showed the relative low incidence. These disparate results are likely related to differences in the cumulative administered dose of 131I and follow up durations used in the various studies, but sucking on lemon candies after 131I therapy in this study might affect the low incidence, as shown in previous reports [20].
All three SGS-based objective scores of salivary gland dysfunction (uptake scores, %WR scores, and functional scores) developed in this study were reduced after 131I therapy, with the scores being the lowest in the high-dose group (generally, high-dose group < low-dose group < pre treatment group). Our results are in agreement with previous studies showing a positive correlation between the cumulative administrated 131I dose and the degree of salivary gland dysfunction [14, 18]. It has been suggested that radiation injury in both the PG and SMG by 131I therapy depends on the cumulative dose of 131I. Goolden AW et al., [22] measured the salivary and plasma concentrations of 131I, and estimated that the radiation dose to the salivary glands was approximately 7 Gy during the first 12 hours of therapy from doses of 3700–7400 MBq. Henriksson R et al., [23] reported that radiation exposure increases the amount of mast cell and hyaluronic acid-mediated damage and the loss of serous acinar cells in the salivary glands, thereby reducing saliva production. Further, absorbed tissue doses between 7 Gy and 15 Gy could induce significant tissue inflammation and ductal obstruction, thus reducing salivary flow [24].
The SGS scores were also correlated with the presence of dry mouth symptoms. All three scores were reduced in both salivary glands when dry mouth symptoms were present (generally, symptom-positive group < symptom-negative group <pre treatment group). The degree of 99mTc-pertechnetate uptake in salivary glands indicates the capability of saliva production and %WR in SGS expresses saliva clearance from salivary glands to the oral cavity [21]. Sodium iodine symporters expressed on the surface of salivary gland cells incorporate iodine inside the cells and induce radiation exposure due to administered 131I [25]. Also, the retention of radioiodine in the intralobular ducts of the salivary glands induces inflammation of the salivary gland duct wall and leads to poor saliva clearance due to narrowing of salivary gland ducts [5, 26]. Thus, poor saliva production and clearance associate with dry mouth symptoms and we think that the scoring system in the present study can concisely assess side effects in salivary glands after 131I therapy. In addition, all three scores in PG were significantly reduced in symptom-negative group, compared with pre treatment group. The reduced scores that were observed in the symptom-negative patients suggest that SGS can detect salivary gland dysfunction by 131I therapy before the development of symptoms. As salivary gland protection, amifostine has been reported to prevent salivary gland damage after administration of high 131I dose [27]. Recently, sialoendoscopic intervention by dilating salivary ducts has also been reported as treatment for chronic sialadenitis [28, 29]. However, it is difficult to obtain successful outcomes in total obstructive cases [28]. Early detection of salivary gland dysfunction by functional PG scores may contribute to determination of indication on such treatment for chronic sialadenitis.
In terms of the strength of the association and predictive value, the PG and SMG functional scores were independent risk factors for dry mouth symptoms and had the highest predictive value among the analyzed factors. As the uptake score reflects saliva production and the %WR score reflects salivary flow and secretion, we posit that the combined functional score better reflects the overall function of the salivary gland and therefore can be used as a feasible and simple method for evaluating salivary gland function secondary to 131I therapy. On the other hand, the cumulative 131I dose was not an independent risk factor for dry mouth symptoms. Some previous papers have reported that individual variations of 131I uptake in salivary glands may influence the incidence of complication in PG [24, 30]. We speculate that individual differences of radiosensitivity in salivary glands as well as administered cumulative 131I dose can associate with dry mouth symptoms.
Further, comparisons between the symptom-negative group and pre treatment group revealed different trends in the PG and SMG. All three scores in the PG were significantly reduced, but all three scores in the SMG were not significantly different between the symptom-negative and pre treatment groups. These results suggest that the PG is likely more sensitive to radiation than the SMG. The difference in radiosensitivity between the PG and SMG could be explained by the higher concentration of serous acinar cells in the PG [24] compared to the mucinous gland-rich SMG, because the serous cells have greater ability to concentrate iodine than mucous cells [5, 31]. Since SMG saliva production is not affected in symptom-negative patients and since the SMG produces nearly two thirds of the total saliva under the daily unstimulated state [27], dry mouth symptoms do not manifest despite PG dysfunction. These mechanisms could also explain the significantly higher predictive value of the SMG functional score compared to the PG functional score for dry mouth symptoms.
Limitation
Our study has several limitations. First, the SGS examination was performed approximately one year after the initial 131I therapy. As salivary gland dysfunction is also known to manifest at later periods, the follow up duration in our study may not have completely captured all cases of salivary gland dysfunction. Second, we did not analyze the amount of saliva in the mouth as an objective measure of salivary gland dysfunction. Third, a decrease in %WR of >10% was defined as salivary gland dysfunction on SGS. We selected the cut off level of 10% according to previous articles [15], but we did not systemically investigate or validate whether the cut off level of 10% is the most optimal value. Finally, the present study is a retrospective study without consecutive cases and did not necessarily have a sufficient sample size for multivariate logistic regression analysis because of excluding DTC patients without undergoing SGS. Therefore, further studies are required to verify practical effectiveness of the functional scoring system.
Conclusion
This study showed that SGS could detect salivary gland dysfunction before the onset of symptoms. Dysfunction in the PG appears to occur before dysfunction in the SMG after 131I therapy. Unlike the cumulative administered 131I dose, the PG and SMG functional scores were independent risk factors for dry mouth symptoms, with the highest strength of association and predictive value. Therefore, we believe that clinicians should use the SGS-based PG and SMG functional scores as objective measures of salivary gland dysfunction secondary to 131I therapy in patients with DTC for timely and efficient management of complications.