Alzheimer’s Disease (AD) is a type of neurodegenerative disorder associated with cognition and memory deficits due to accumulation of amyloid plaques and neurofibrillary tangles along with disruption of cholinergic neurons in the basal forebrain of aged people above 65 years [1]. Cholinergic degeneration describes that cognitive decline is associated with AD pathogenesis [2], which also proposes that deficit of Acetylcholine is life-threatening in creation of the symptoms of AD [3]. Therefore, a major achievement in the treatment of AD involves preventing the degeneration of cholinergic neurons of the brain and increase the Acetylcholine level in the central nervous system. In addition, several investigations demonstrated that Reactive Oxygen Species (ROS) produce a pattern of cumulative damage of cellular macromolecules and impairment of mitochondrial function with age leading to a decrease in cellular energy production [4] and excessive level of ROS which are associated with the aetiology of AD [5].
In view of the above, the present investigation was focused to assess the anti-Alzheimer properties of acetylcholine-producing L. plantarum MTCC1325 against D-Galactose induced Alzheimer’s disease in albino rats through its potential antioxidant nature and bi-directional communication between gut-brain axis.
Materials and Methods
The present experiments were planned from the month of February 2015 to June 2015 on the albino rats (wistar strain) and were maintained in the lab as per the guidelines of Institutional Animal Ethical Committee (Resolution No. 34/20122013/(i)/a/CPCSEA/IAEC/ SVU/KY dt.01.07.2012).
Chemicals Associate
In the present study, D-Galactose, acetylcholine iodide and DeMan Rogosa and Sharpe (MRS) broth were procured from Hi-Media chemicals (Mumbai, India), Di Thiobisnitrobezoic Acid (DTNB) and hydroxylamine hydrochloride from Sisco Research Laboratory Mumbai, India, Ferric chloride from Qualigen Chemicals Mumbai, India and sucrose from Sd-fine chemicals, Mumbai and chemicals of high grade quality were procured from the above companies.
Collection of Lactic Acid Bacteria
Pure culture of Lactobacillus plantarum MTCC1325 was obtained from the IMTech, Microbial Type Culture Collection Centre, Chandigarh, India.
Preparation of L. plantarum Culture for Treatment and Experimental Design
L. plantarum MTCC1325 was found that, this strain had the ability to produce acetylcholine neurotransmitter [17]. Preparation and treatment of L. plantarum MTCC1325 was done according to our previously reported studies (12×108 CFU/ml; 10ml /kg body weight) [13]. In the present investigation, forty eight healthy male wister rats (3 months old and mean weight 180±20 g) were divided into four groups, each group consisting of six animals.
Group-I (Control): received normal saline (1 ml/kg body weight);
Group-II (AD-Model group): received intraperitoneal injection of D-Galactose (120 mg/kg body weight);
Group-III (Protective group; AD+L.P): From seventh week onwards received both D-Galactose and L. plantarum (10 ml/kg body weight; 12×108 CFU/ml) simultaneously for another 60 days;
Group-IV (L.P group): From seventh week onwards received L. Plantarum for 60 day.
Observation of Morphological Features
Morphological features such as general appearance, body weights [18] and organ index [19] were recorded in all groups of rats throughout the experimental period. Vital organs viz., brain, spleen, kidney and liver were weighed and their weights relative to the final body weight (organ weight) were calculated.
Evaluation of Cognitive Behaviour
Morris R [20] performed water maze experiment to assess the cognitive function in all groups of rats on two seleted days i.e., 30th and 60th day after treatment with L. plantarum MTCC 1325. Administration of D-Galactose and L. plantarum MTCC 1325 was continued during the entire period of the water maze experiment.
Assessment of Gross Behavioural Activity
The animal behavioural activity was observed on the selected days i.e., 30th and 60th day for each animal in all four groups. Each animal was placed in a square (30 cm) closed area equipped with infra-red light sensitive photocells using digital actophotometer [21]. The animal was observed for a period of five minutes and values were expressed as counts / five minutes. The apparatus was placed in a darkened, light, and sound attenuated and ventilated test room.
Isolation of tissues: After six weeks of Alzheimer’s disease induction period, the animals were treated with L. plantarum MTCC1325 for a period of 60 days. Further, the animals were kept fasting for 12 hours before sacrificing them by cervical decapitation on selected days of experimentation i.e., 30th and 60th days. Selected brain regions such as hippocampus and cerebral cortex from each animal was rapidly isolated, thoroughly washed with ice cold saline and then stored at -800C for biochemical analysis.
Histopathological Examination
Brains, fixed in neutral-buffered 10% formalin, sectioned and stained as per manufactures protocols with Congo red stain (Nova Ultra Congo-Red Stain Kit, IHC World Science Products and Services, USA) were used specifically for observation of the amyloid protein aggregation. Histopathological examinations for all groups were observed under a light microscope.
Biochemical Estimation of Cholinergic System
Acetylcholine (ACh) was estimated by the method of Metcalf [22] as given by Augustinsson KB [23] and was expressed as μ moles of ACh/g wet weight of tissue. Acetylcholinesterase (AChE) was estimated following the method of Ellman GL et al., and expressed as μ moles of ACh hydrolysed/mg protein/hour [24]. Protein content of the brain tissue was estimated by the method Lowry OH et al., [25].
Statistical Analysis
All the experiments were carried out in triplicates and values of the measured parameters were expressed as Mean±SEM. One-way ANOVA was used to test the significance of difference among four different groups followed by Dunnet’s Multiple Range Test (DMRT). Statistical analysis was performed by using Statistical Program of Social Sciences (SPSS) for windows (Version 20.0; SPSS Inc., Chicago, 1L, USA). The data were regarded as statistically significant different at p<0.05.
Results
Morphological Features
Morphological features such as general appearance, body weight and organ index were observed in all groups of rats during the entire experimentation. When compared with the control group, AD-model rats showed gradual loss of hair, skin elasticity which became stiff, thin and saggy. Further, during AD induction period, the body weight of rats gradually decreased when compared to the control group shown in [Table/Fig-1]. Simultaneous treatment of AD group with L. plantarum MTCC 1325 i.e., the protective group (AD+L.P) showed a significant increase in total body weight, when compared with the AD-model group.
Effect of L. plantarum MTCC 1325 on body weight of experimental and control groups of rats. Values are mean ± SEM (n=6). *p<0.05 vs. Control; #p<0.05 vs. AD model group.
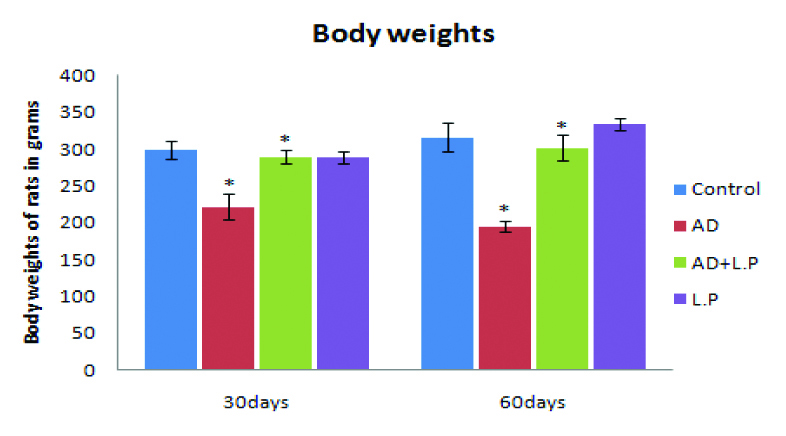
As a result, protective group rats treated with L. plantarum MTCC 1325 for 30 and 60 days exhibited increased body weight when compared to AD-model group (289.66±8.63 vs. 222.2±17.19 and 301.26±17.42 vs. 195.32±7.59) respectively. Beside the control group, LP groups have also recorded a similar trend. Organ weight in grams for selected tissues, namely include brain, spleen, kidney and liver was calculated as average mean values (Mean±SD) from organs isolated from six animals from each group. The organ index showed significant decline (p<0.05) in the AD-model group than that in the L. plantarum treated group and control group [Table/Fig-2]. Nevertheless, the organ index of protective group exhibited better gain in organ weight than that of AD-model group.
Organ index from different groups of experimental rats for 30 and 60 days treatment.
| Parameter | Brain (g) | Kidney (g) | Liver (g) | Spleen (g) |
|---|
| Group | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days |
|---|
| Control | 1.86±0.34 | 1.98±0.11 | 1.96±0.19 | 1.98±0.22 | 9.28±0.53 | 9.81±1.26 | 1.29±0.11 | 1.19±0.25 |
| AD | 1.63±0.20 | 1.67±0.19 | 1.82±0.31 | 2.03±0.16 | 7.45±0.16 | 8.66±1.12 | 0.88±0.11 | 1.15±0.29 |
| AD+L.P | 2.03±0.10 | 1.89±0.09 | 2.20±0.21 | 2.38±0.29 | 8.93±0.94 | 10.10±1.71 | 1.30±0.17 | 1.35±0.39 |
| L.P | 1.86±0.07 | 2.0±0.13 | 2.02±0.18 | 2.09±0.11 | 10.13±1.30 | 10.41±0.95 | 1.70±0.28 | 1.64±0.19 |
Note: The above values are expressed in grams.
Cognitive Behaviour
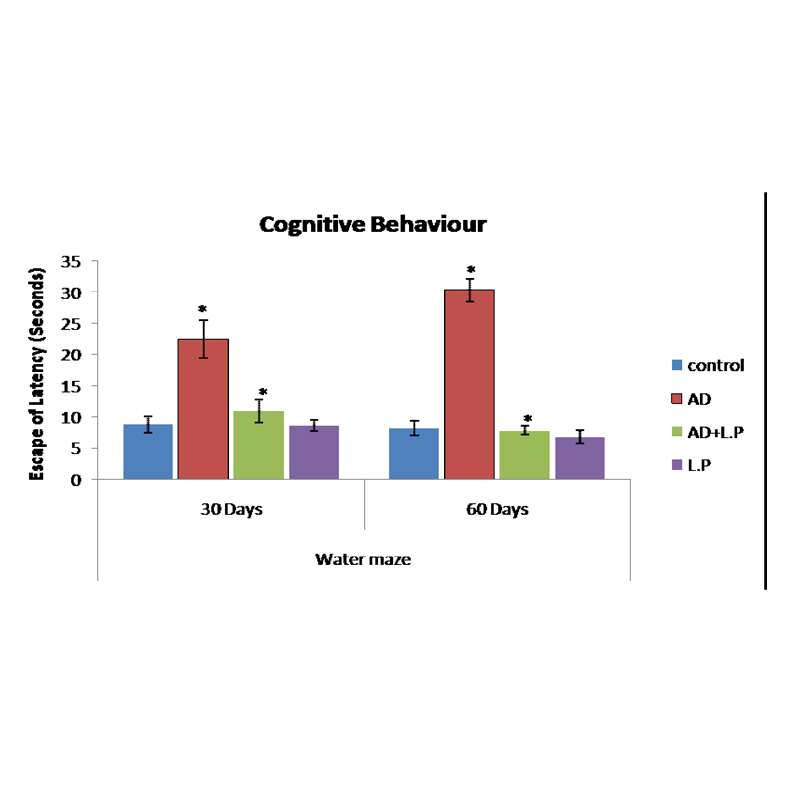
The modulatory effect of Lactobacillus plantarum MTCC1325 on spatial memory impairment in rats induced by D-Galactose was studied by Morris water maze experiment. Data presented in the [Table/Fig-3] showed that, the mean escape latency of AD model group was longer than the control group, while the protective group (AD+L.P) took a significantly shortened escape latency.
Effect of L. plantarum MTCC 1325 on cognitive performance on selected days in different groups of experimental rats against the control. Values are mean±SEM (n=6). *p<0.05 vs. control; #p<0.05 vs. AD model group.
Gross Behavioural Activity
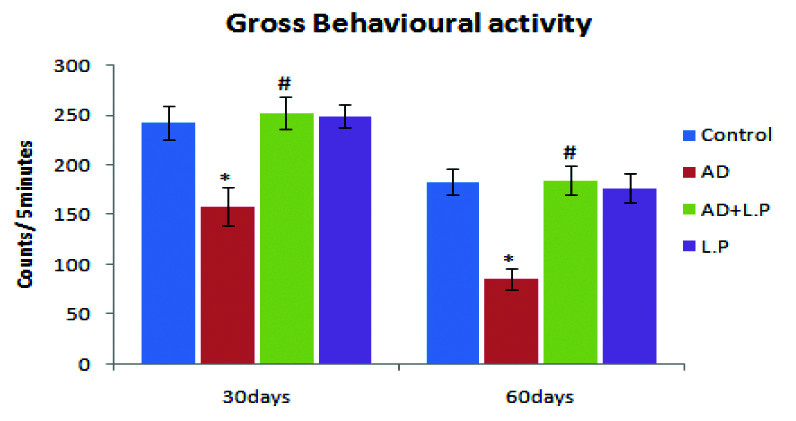
From the results obtained on the gross behavioural activity, it was obvious that AD-model rats showed significant decrease in their activity when compared with control group [Table/Fig-4]. At the same time, as compared with the AD-model group, (protective group) L. plantarum MTCC 1325 treated groups have revealed significant improvement in the activity of rats when compared with the control group.
Effect of Lactobacillus plantarum MTCC 1325 on gross behavioural activity on selected days in different groups of experimental rats against the control. Values are mean±SEM (n=6). *p<0.05 vs. Control; #p<0.05 vs. AD model group.
Histopathological Examination
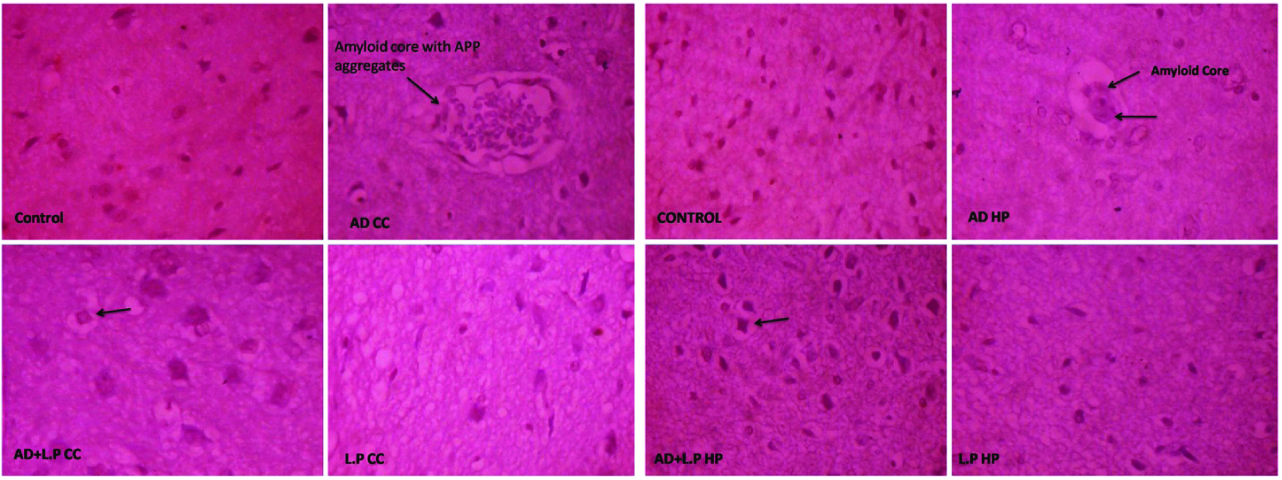
The results of histopathological examination clearly demonstrated that intraperitoneal injection of D-Galactose in AD-model group rat brain regions such as hippocampus and cerebral cortex showed beta-amyloid plaques for 30 days followed by neurofabrillary tangles were also observed on the 60th day [Table/Fig-5,6].
Sections of cerebral cortex and hippocampus regions of brain from different experimental groups of rats against the control for 30 days treatment. (Congo Red Stain, Light Microscope: 40X). {AD CC: AD-model group Cerebral Cortex; AD+L.P CC: Protective group Cerebral Cotex; L.P CC: Lactobacillus plantarum Group Crerebral Cortex, AD HP: AD-Model group Hippocampus; AD+L.P HP: Protective group Hippocampus; L.P: Lactobacillus plantarum group Hippocampus}.
Sections of Cerebral Cortex and Hippocampus regions of the brain from different experimental groups of rats against the control for 60 days treatment. (Congo Red Stain, Light Microscope: 40X). {AD CC: AD-model group Cerebral Cortex; AD+L.P CC: Protective group Cerebral Cotex; L.P CC: Lactobacillus plantarum Group Crerebral Cortex, AD HP: AD-Model group Hippocampus; AD+L.P HP: Protective group Hippocampus; L.P: Lactobacillus plantarum group Hippocampus}.
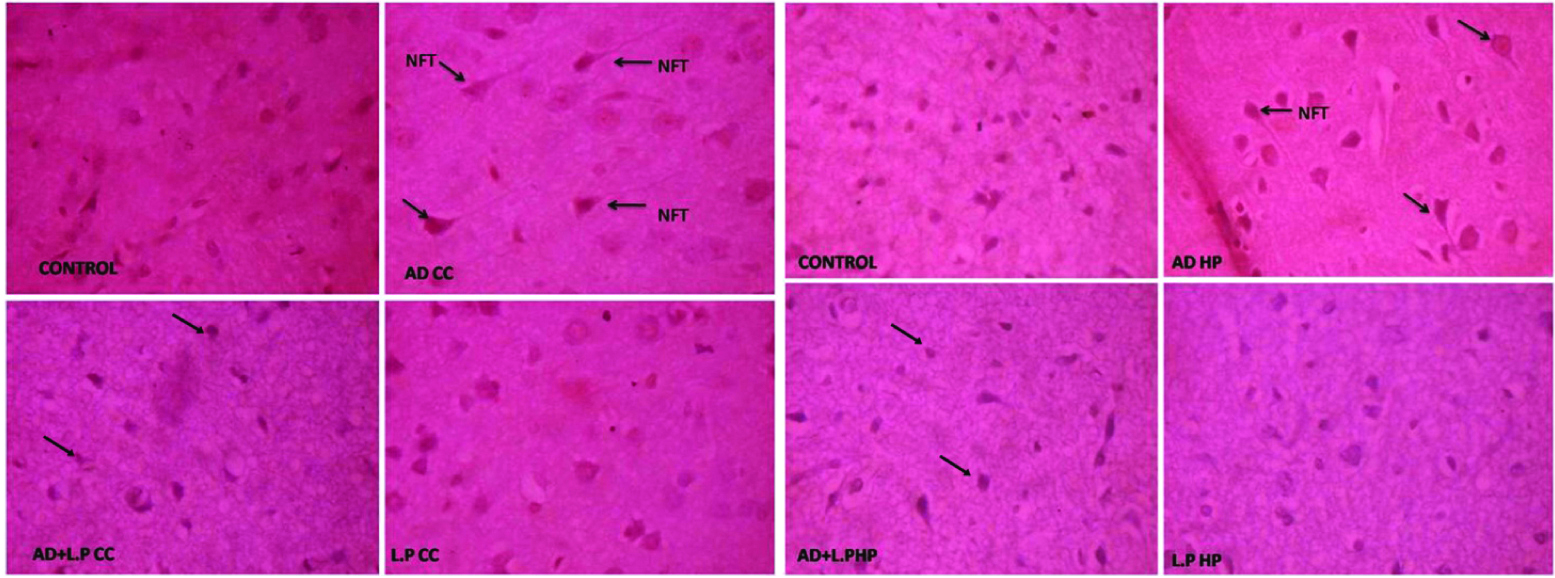
Beta-amyloid proteins aggregated with amyloid core and neurofabrillary tangles appeared as long pink filament in the cytoplasm. Protective group (AD+L.P) showed healthy neurons with hyper chromatic nuclear chromatin. However, some partially degenerated neurons were also observed on the 30th day onwards, and on the 60th day considerable recovery was observed, evidenced by clearly healthy neurons with prominent nucleai. In case of L. plantarum treated and control groups, healthy neurons with prominent nuclei were observed.
Cholinergic System (ACh and AChE)
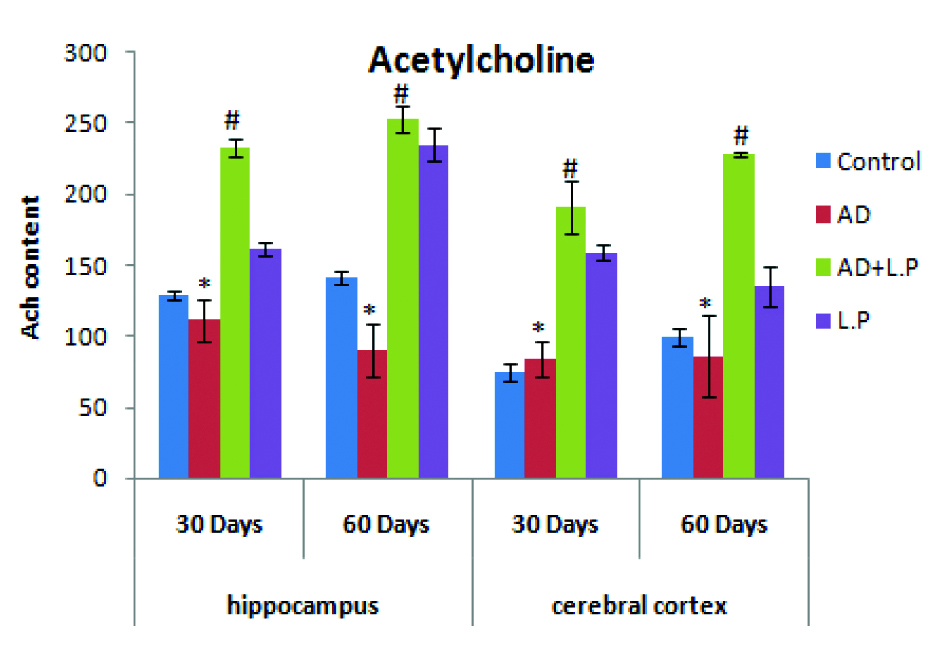
As compared with control group rats, the ACh content significantly declined in the cortex and hippocampus of AD-model group rat brain [Table/Fig-7]. Contrary to this, a significant increase in ACh levels was observed in protective group (AD+L.P) for the selective days of experimentation. Treatment with L. plantarum MTCC 1325 showed a significant elevation of ACh in both cerebral cortex and hippocampus was observed. In contrast to ACh, D-Galactose treatment for six weeks caused a significant increase in Acetylcholinesterase (AChE) activity as compare to control group.
Effect of Lactobacillus plantarum MTCC 1325 on the levels of acetylcholine in hippocampus and cerebral cortex regions of control and experimental groups of rats at selected intervals. Values are mean±SEM (n=6). *p<0.05 vs. Control; #p<0.05 vs. AD model group.
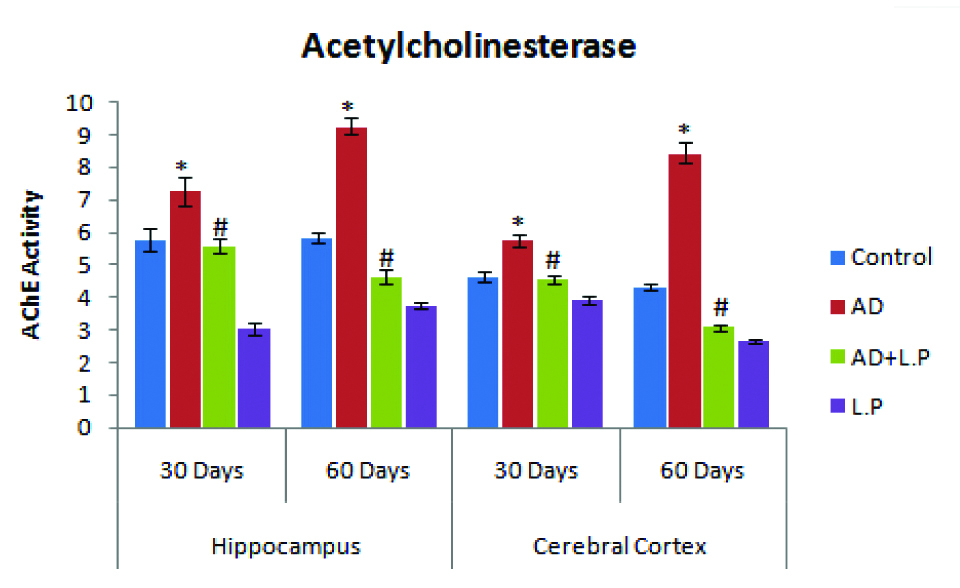
However, chronic oral administration of Lactobacillus plantarum MTCC1325 showed significantly decreased Acetylcholinesterase as compare to the AD-model group. However, L. plantarum MTCC1325 treatment alone could not exert any effect on AChE activity as compare to control group [Table/Fig-8].
Effect of Lactobacillus plantarum MTCC 1325 on the levels of Acetylcholinesterase in hippocampus and cerebral cortex regions of control and experimental groups of rats at selected intervals. Values are mean±SEM (n=6). *p<0.05 vs. Control; #p<0.05 vs. AD model group.
Discussion
In this preliminary study, we have chosen the strain, L. plantarum MTCC1325 to evaluate its ameliorative effect against Alzheimer’s disease. In our present study, the result strongly supported by earlier reports describing the chronic injection of D-Galactose induces memory impairment, neurodegeneration and oxidative damage in mice [26]. L. plantarum MTCC 1325 has the ability to produce the neurotransmitter i.e., acetylcholine [17]. Biochemically, it was well established that AD has been related to a significant decrease in the ACh neurotransmitter [27] and oxidative stress, eventually leading to imbalance production and detoxification of ROS which is considered as one of the important factors for the development of AD. During the entire tenure of our experiment, AD-model rats showed a significant decline in total body weight, organ index, hair loss, skin elasticity [28]. Further, chronic administration of D-Galactose caused significant decline in spatial memory and reduced gross behavioural activity suggesting impairment of memory [29].
Our observations on Morris water maze test, showed significant differences between control, AD-model and protective group’s rats suggest that oral administration of L. plantarum MTCC 1325 might ameliorate memory impairment and behavioural changes in AD-model rats. Our results derive strong support from earlier reports where in Lactobacillus pentosus var. plantarum C29 showed significant improvement in memory impaired mouse induced by scopolamine and D-Galactose [11,12]. It was also described that L. plantarum NDC 75017 alleviate learning and memory associated injuries in ageing rats by reducing the mitochondrial dysfunction induced by D-Galactose [13].
From the comparative studies conducted on the above aspects on 30th and 60th day in all groups of rats, it was evident that chronic administration of L. plantarum MTCC 1325 for 60 days showed significant improvement and recovery from AD, which is related to duration of AD induction and treatment period with L. plantarum. However, AChE level was brought back to near normal conditions. Similarly, elevated levels of AChE, characteristic features of cognitive deficit was also been reversed to normal levels on treatment of AD-model rats with L. plantarum. Similarly, the results on the cholinergic system indicated that chronic administration of D-Galactose caused a significant reduction in ACh level in brain due to dysfunction of cholinergic neurons and reduced activity of ChAT [30]. Contrary to this treatment with L. plantarum MTCC 1325 significantly elevates the ACh content in both the regions of brain such as hippocampus and cerebral cortex. This might be associated with the potential antioxidant nature as well as Acetylcholine producing activities of L. plantarum MTCC 1325 and also bi-directional communication between the gut-brain axis (Enteric Nervous System) [14-16,27]. This was supported by the recent reviews [10,31,32] on gut-brain axis communication describing that the bacteria (microbiome) present in the gastro-intestinal tract may communicate with brain and nervous system by different ways and stimulate the production of ACh in both hippocampus and cerebral cortex with L. plantarum treatment. Most of the gut microflora produces neurochemicals are called neurotransmitters, which are similar in their structures and functions of the host nervous system and modulate the behaviour and mood [31].
Microbes also have exhibited immunomodulatory effect by the release of host immune factors such as cytokines and inflammatory mediators that have known neuronal targets both in the Central Nervous System (CNS) and Enteric Nervous System (ENS) [33]. Another way is that most of the lactic acid bacteria and probiotics may activate the vagal nerves, the largest 10th cranial nerve that interact with all neurons involved in alleviation of behavioural changes like anxiety, learning and memory, depression etc., [34]. It has been demonstrated that a probiotic bacterium, L. rhamnosus JB-1 influenced emotional behaviour in mice mediated via GABA receptor [32]. Recently, lactic acid bacteria such as L. pentosus var. plantarum C29 were shown to be involved in the up regulation of Brain Derived Neurotrophic Factor (BDNF) protein expression in both D-Gal and Scopolamine-induced senescent and improved from the toxic effect of the chemical [11,12]. The present study is at its preliminary stage and the precise mechanism underlying the relationships between the L. plantarum MTCC1325 and Alzheimer’s disease need further investigations.
Limitation
Limitation of the present study is designed only for morphometric, behavioural, cholinergic and histological aspects involved in the Alzheimer’s disease. The remaining parameters including various biochemical changes and gene expression studies should be performed.
Conclusion
From the results obtained in our investigation, it was finally inferred that, antioxidant and ACh producing L. plantarum MTCC 1325 has anti-Alzheimer properties against D-Galactose induced Alzheimer’s disease since it resulted in body weight gain and organ index, improved the behavioural activity and learning skills through elevation in the cholinergic neurotransmitter in hippocampus and cerebral cortex regions of brain and restored histopathological abnormalities back to the normal conditions etc. All these preliminary findings suggested that, the L. plantarum MTCC 1325 might have exerted ameliorative effect against Alzheimer’s disease induced by D-Galactose.
Financial Support
The researcher, Mr. N. Mallikarjuna thanks the UGC, New Delhi for its financial support through BSR Fellowship to carry out this work.