Magnetic Resonance Imaging (MRI) is fast emerging as a powerful tool for evaluation, treatment and response assessment in cervical cancer. The importance of MRI in pretreatment evaluation has been recommended by several recent guidelines [1–3]. In locally advanced disease, MRI is useful in determining the tumour size, craniocaudal extent, loco-regional infiltration like involvement of lower uterine segment, bladder and rectum and is superior to CT scan in detecting parametrial invasion [4]. DWI is based on the Brownian movement of water molecules. This movement of water molecules is influenced by change in cellularity, integrity of cellular membranes, tortuosity of extracellular space, and viscosity of tissue fluids which is reflected in the signal intensity of the imaging. DWI provides a superior tissue contrast compared to T2HR images and is useful to differentiate tumour from necrosis or post radiotherapy changes [5–7]. ADC, a parameter derived from DWI, has shown potential as an imaging biomarker for clinicopathological features, nodal metastasis [8–10] and response assessment [6,7,11–13]. The ADC value in the case of cervical cancer is significantly lower than in the non-affected cervical tissue [14]. Change of ADC value has a potential role as an imaging biomarker in prediction of response [11,13,15] and outcomes of cervical cancers [13,16–20]. It has been shown to be a useful tool in monitoring and evaluation of response [21–24]. Clinicopathological correlation of this imaging biomarker in cervical cancer is an area of translational research and evidence is evolving regarding its role in locally advanced disease in developing countries where cervical cancer is a major public health problem [19]. The aim of this retrospective study was to analyse the role of functional magnetic resonance imaging derived parameters as imaging biomarkers and their correlation with clinicopathological features in carcinoma of uterine cervix.
Materials and Methods
This retrospective study was conducted in Christian Medical College, Vellore, Tamil Nadu, India, and was approved by the Institutional Review Board. Women diagnosed to have cervical cancer between October 2012 and November 2016 who had a baseline MRI scan were included in the study.
Pretreatment Evaluation
Pretreatment evaluation included detailed history, physical examination including pelvic examination, four quadrant cervical punch biopsy, haemogram and biochemical parameters including renal function test and liver function test, ECG, echocardiogram, chest X-Ray, ultrasound abdomen and MRI pelvis. Cystoscopy was considered in selected patients if bladder involvement was suspected. Patients were clinically staged using FIGO classification (2009 version) [25].
Treatment Protocol
In patients who underwent radical chemoirradiation, radiotherapy delivered with megavoltage teletherapy machines (linear accelerator using 6MV/15 MV energy or cobalt 60 machine). A total dose of 80 – 85 Gy (1B2 – IIB) or 85 – 90 Gy (III A – III B) to point A (defined as 2 cm superior to the external cervical os and 2 cm lateral to cervical canal) was delivered using combination of external beam radiotherapy (50 Gy in 25 fractions) and intracavitary HDR (high dose rate brachytherapy) with a dose of 30–35 Gy (LDR, low dose rate equivalent) in 2–3 fractions to point A. Concurrent cisplatin at a dose of 40 mg/m2 was administered along with radiotherapy once a week for 4–5 cycles.
In adjuvant radiotherapy settings, the dose of radiotherapy was 50-50.4 Gy in 1.8–2 Gy per fraction followed by two fractions of brachytherapy using vaginal cyinders (6 Gy each fraction prescribed to 0.5 cm depth).
In the neoadjuvant chemotherapy regimen patients received three cycles of chemotherapy with paclitaxel (175 mg/sq.m) and carboplatin (5 AUC) followed by definitive treatment.
Surgery, radical hysterectomy and bilateral lymphadenectomy were done and adjuvant therapy was given according to the risk factor stratification.
Patients were followed up once in three months in the first year, once in six months in the second and third year and once a year in the subsequent years. During follow up, apart from clinical examination, haemoglobin, creatinine, chest X-ray, ultrasound abdomen and pelvis were done. If the patients had clinical or radiological suspicion of residual/recurrence, biopsy was advised along with volumetric imaging (CT/MRI with DWI/PET) at physician’s discretion.
Imaging Protocol and Derivation of Apparent Diffusion Coefficient
MRI pelvis including diffusion images were acquired by a 3.0 Tesla MRI scanner (Achieva, Philips Healthcare, Netherlands). In brief, the following sequences were done in all patients: T2 weighted coronal and axial sequences using a large Field Of View (FOV) including the pelvis from below aortic bifurcation; Short Tau Inversion Recovery (STIR) and T1 weighted axial large FOV images; High Resolution (HR) T2 weighted imaging using small FOV in three planes (axial, coronal and sagittal with respect to the plane of the uterine cervix); Diffusion weighted imaging with b-values of 0 and 800 sec/mm2. ADC maps were generated and Region Of Interest (ROI) with minimum size of 10 mm2 was drawn manually within the tumour showing restricted diffusion. Mean ADC value was calculated as the average of the three ADC values from each ROI (units -10-6 mm2/sec). In addition, the minimum ADC value of the tumour was noted (ADC min). Tumour size was recorded in three orthogonal planes – transverse, antero-posterior and sagittal – with respect to the orientation of the cervical tumour. Pelvic nodes (≥10 mm or highly suspicious if <10 mm) were taken as significant.
Tumour Volume Delineation in MRI and DWI
The tumour was manually outlined on each section of the T2 weighted high resolution axial images and diffusion images. While using DWI images, only tissue which showed restricted diffusion i.e., hyperintense on DWI and hypointense on ADC map was outlined and used for calculating tumour volume. The volume of the tumour was calculated by multiplying the sum of areas of all sections by sum of slice thickness and inter-slice gap using automated volumetric tool of Eclipse treatment planning system (Eclipse TPS TM version 10, Varian Medical Systems Inc., Palo Alto, USA).
Statistical Analysis
Statistical analysis was done by SPSS software version 15.0. Mean, standard deviation, kurtosis, skewness of ADC parameters were calculated and means were compared by independent sample t-test. In all cases, p<0.05 was taken as statistically significant. Kaplan Meier curve was plotted for disease free survival and overall survival in patients with recurrence.
Results
Patient, Tumour and Treatment Related Characteristics
The mean age of the cohort was 50 years (range: 28-70, n= 100). The most common presenting symptoms were vaginal bleeding (n=71), white discharge (n=20), abdominal pain (n=2) and low backache (n=1). Six patients complained of both bleeding and white discharge. The mean pre treatment haemogobin was 10.92 (range: 4.0 -15.0). Patient, tumour and treatment characteristics are presented in [Table/Fig-1].
Patient, tumour and treatment related characteristics.
| Characteristics | Frequency (Percent) N=100 |
|---|
| Stage1b12A2B3A3B4A | 117541261 |
| PatternInfiltrativeUlcerativeUlceroproliferativeIrregularity | 237682 |
| HistologySquamousAdenoAdenosquamousCarcinoma | 88642 |
| DifferentiationWellModeratePoor | 262846 |
| Lymphovascular invasionPresentAbsent | 1189 |
| Treatment detailsChemo irradiationChemotherapySurgeryChemotherapy – surgeryRadiotherapyChemotherapy →chemoradiotherapySurgery→ radiationDefaulted treatment | 722315188 |
| RecurrenceLocalNodalDistant | 16646 |
Seventy two patients received chemoirradiation, eight patients had surgery followed by radiotherapy, five patients received radiotherapy alone, three patients had surgery alone, two patients received chemotherapy, one patient had chemotherapy followed by surgery and another one patient had chemotherapy followed by chemoirradiation. Eight patients did not continue treatment after the diagnosis. Follow up details of ten patients were not known inspite of best efforts (two patients treated with chemotherapy and eight patients defaulted after diagnosis).
Three patients had persistent disease at first follow up at three months (residual disease). Thirteen patients had disease recurrence either locoregional (n=7) and distant (n=6). MRI including DWI (n=2), PET CT scan (n=5) or CT scan with contrast (n=9) was done if recurrence was suspected. Six patients had biopsies from the recurrent site of the disease.
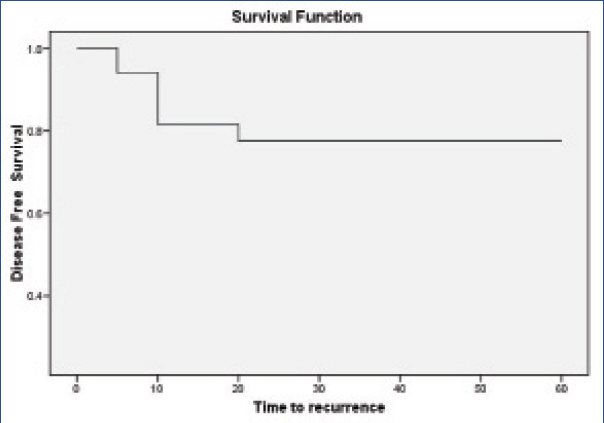
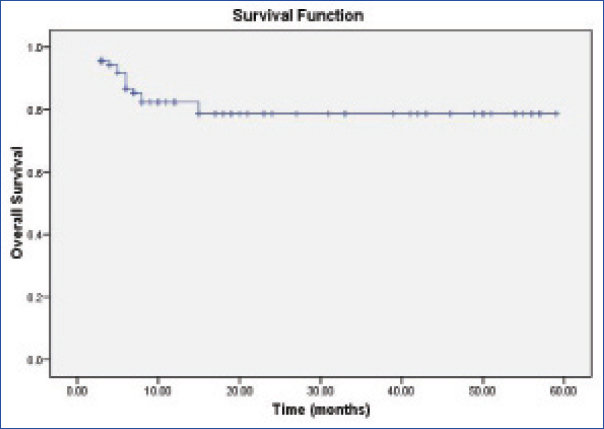
For calculating disease free survival both residual/recurrent disease were taken into account. The median time to recurrence was six months (3-15 months, n=16). Cumulative survival was 78% at a median follow up of 12 months (3-59 months, n=100, [Table/Fig-2a,b])
The Kaplan Meier curve for disease free survival in patients with recurrence.
The Kaplan Meier curve for overall survival in patients with recurrence.
The mean tumour dimensions on MRI in x, y and z axes were 43.04 mm (±13.93) (range: 17-85), 37.05 mm (±11.83, range: 9-80) and 39.63 mm (±14.81) (range: 14–76). The mean T2W MRI based GTV was 48.18 (±34.3, range: 7–206) and on DWI images was 36.68 (±33.72, range: 2.5–200).
Volumetric and Functional Parameters
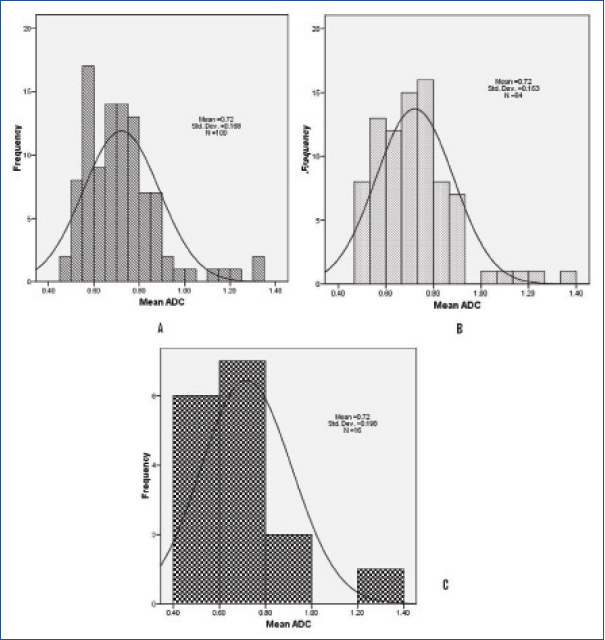
The mean ADC values displayed a normal distribution (population mean: 0.72 (±0.168, range: 0.49–1.35, [Table/Fig-3]). The population mean of the minimum ADC values was 0.524 (±0.149, range: 0.23–1.15). The mean ADC value in patients with squamous cell carcinoma (n=88) was 0.694 (±0.125), adenocarcinoma (n=6) was 0.989 (±0.309), adenosquamous (n=4) was 0.894 (±0.324). There was significant statistical difference in mean ADC between squamous versus non squamous histology (p=0.02). The mean ADC values of well differentiated (n=26), moderately differentiated (n=28), poorly differentiated tumours (n=46) were 0.841 (±0.227), 0.729 (±0.125), 0.648 (±0.099) respectively. There was significant statistical difference between well differentiated, moderately differentiated (p=0.020) and poorly differentiated tumours (p=0.001). The mean ADC values in infiltrative, ulcerative, ulcero proliferative lesions were 0.72(±0.168), 0.664 (±0.108) and 0.726 (±0.175) respectively (p=0.97, infiltrative vs. non infiltrative). The other ADC values in relation with tumour parameters are presented in [Table/Fig-4]. Difference between the mean ADC values between the node positive and node negative disease was statistically significant (p=0.001). The skewness and kurtosis calculated for mean ADC values for squamous cell carcinoma, adenocarcinomas were 0.816, 1.357, 0.252 and -1.115 respectively. Skewness for well, moderately and poorly differentiated tumours was 0.954, 0.118 and 0.466. The kurtosis was 0.180,-0.543 and -0.355 respectively. There was no correlation between the tumour volumes on T2W and DWI images and ADC values. Sixteen patients had recurrence (local recurrence – 6, nodal recurrence – 4 and distant metastases – 6). Remaining 84 patients were on follow up. The patients who had disease failure had significantly large tumour volumes both on T2W MRI and DWI. There was no significant difference between the ADC values of recurrence versus no recurrence group [Table/Fig-5].
Distribution of mean ADC (n=100, A). Mean ADC in patients with no recurrence (n=84, B), with recurrence (n=16, C).
Mean ADC values in relation to tumour extent and spread.
| Parameters | N | Mean ADC (present) | Mean ADC (absent) | p-value |
|---|
| Parametrium involvement | N=77 | 0.769(±0.205) | 0.706(±0.153) | 0.11 |
| Nodes present | N=31 | 0.748(±0.168) | 0.678(±0.160) | 0.03 * |
| Lower uterine segment involvement | N=55 | 0.713(±0.150) | 0.738(±0.186) | 0.40 |
| Hydroureteronephrosis present | N=11 | 0.680(±0.890) | 0.726(±0.174) | 0.30 |
| Vaginal involvement | N=81 | 0.716(±0.164) | 0.742(±0.185) | 0.50 |
| Bladder involvement | N=8 | 0.684(±0.101) | 0.724(±0.172) | 0.50 |
| Rectum involvement | N=3 | 0.744(±0.132) | 0.720(±0.169) | 0.80 |
= p-value < 0.05 is statistically significant, Statistical test used: independent sample t-test
Comparison of volumetric and functional parameters in patients with and without recurrence.
| Parameters | Recurrence | No recurrence | p-value |
|---|
| Mean ADC | 0.71 ± 0.19 | 0.72 ± 0.16 | 0.92 |
| Min ADC | 0.49 ± 0.16 | 0.52 ± 0.14 | 0.41 |
| Skewness of mean ADC | 2.1 | 1.3 | NA |
| Kurtosis of mean ADC | 5.1 | 2.9 | NA |
| Tumour volume (MRI) | 67.5 ± 52.0 | 30.8 ± 25.4 | 0.01 * |
| Tumour volume (DWI) | 78.1 ± 45.1 | 42.4 ± 28.8 | 0.007 * |
= p-value < 0.05 is statistically significant, Statistical test used: independent sample t-test
Discussion
Cervical cancer is an important public health problem characterized by high mortality to incidence ratio in developing countries like India. Inspite of advancement in diagnosis and therapy, survival in locally advanced cervical cancer is still dismal [26]. Though concurrent chemoradiation is the standard of care, benefit of addition of chemotherapy is debated especially in locally advanced settings [27]. Updated Cochrane meta-analyses [28] showed that benefit of addition of concurrent chemotherapy to radiotherapy, particularly in stages III-IVA, is minimal (3%). A large proportion of patients in developing countries present in locally advanced stages. Therefore, in the present scenario, prognostication of the disease with non-invasive imaging biomarker is highly relevant translational research. This will help to choose patients for intensification of therapy. Traditionally, clinical examination remains gold standard for evaluation, but MRI is emerging as an important adjunct in diagnosis and management of carcinoma cervix [29], both in early and locally advanced cases [30]. The role of volumetric and functional parameters of MRI, is an active area of research in staging, prognostication, management and follow up [31]. Conventional T2HR MRI along with DWI is useful adjunct in delineating not only the tumour but also the disease extension to lower uterine segment, parametrial involvement [Table/Fig-6,7a,b], vaginal involvement, nodal involvement [Table/Fig-7c,d], involvement of adjacent structures [Table/Fig-8a,b] and differentiating metastatic lesions from benign lesions [Table/Fig-9a,b]. DWI provides a good depiction of the primary tumour, residual disease and helps in assessing response after treatment [29]. This has significant implication not only in diagnosis but also in deciding the optimal therapy in multidisciplinary tumour board.
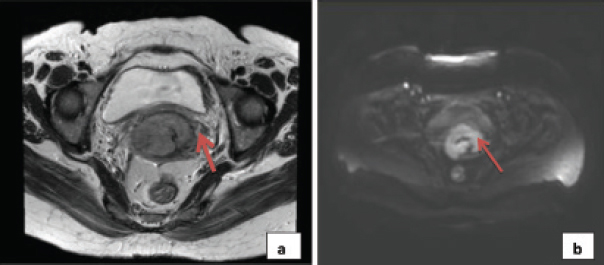
a) Clinically staged as IB1, T2W HR MRI showed lesion in the cervix, measuring approximately 5.1 × 3.8 × 5.5 cm with parametrial stranding, more so on the left side, not extending to the pelvic side wall; b) Corresponding diffusion weighted image showing restricted diffusion in the same region.
a) T2 weighted image showing cervical tumour with left sided parametrial stranding not appreciated in clinical examination; b) Diffusion weighted image showing restricted diffusion in the same region; c) T2 weighted image showing tumour with large right sided obturator node; d) Diffusion weighted image showing restricted diffusion in the nodal region.
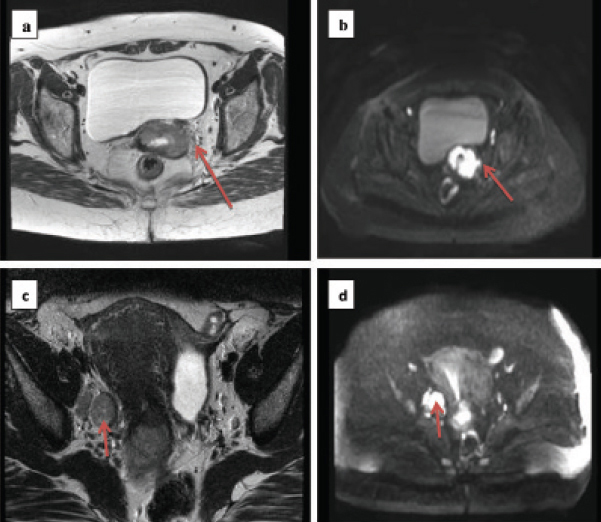
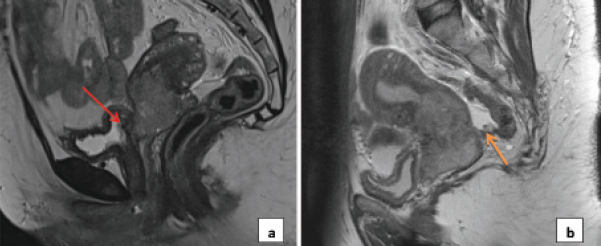
a) Carcinoma cervix with retroverted uterus and bladder involvement; b) Carcinoma cervix with deposit in pouch of Douglas.
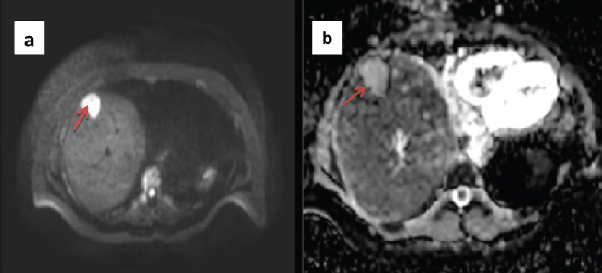
a) Diffusion image showing a bright lesion in the liver in a patient with cervical cancer; b) Corresponding ADC image showing no evidence of restricted diffusion, hence this lesion is most likely to represent atypical hemangioma.
Recently, it has been shown that, ADC related parameters such as pretreatment ADC, post-treatment ADC and absolute change in ADC (ΔADC) and Tumour ADC Increase Rate (TAIR) are potential prognostic biomarkers that can be useful in response assessment [6,7,32]. Distribution of cellular architecture, glandular structures and extracellular space influences the molecular impedance that is reflected by the ADC histogram parameters like central tendency, skewness and kurtosis [33–35]. Our study showed that mean ADC was lower for squamous histology (p=0.02), poorly differentiated tumours (p=0.02), and node negative disease (p=0.03). It has been shown in the literature that, distribution parameters of ADC map like mean, skewness and kurtosis are useful imaging biomarker for clinicopathological factors like histology, differentiation and nodal involvement [34]. Lin Y et al., based on ADC histogram, reported higher median ADC for well/moderately differentiated tumours and less positive skewness in adenocarcinomas [35]. In view of more cystic and glandular spaces in adenocarcinoma compared to squamous cell carcinomas (that has increased cellular density), adenocarcinoma has higher ADC values but less positive skewness. Several authors reported ADC values were significantly lower in patients with lymph nodal metastases [8–10]. We did not find any correlation between ADC and lymphovascular space invasion or tumour volumes though larger tumours were associated with early recurrence (p=0.007). Similar result was reported by Lin Y et al., [35] No association of pre-treatment ADC values with response was noted. This could be because of the fact that absolute change of ADC value (ΔADC) was more relevant for clinical and radiological response than single pretreatment value [32]. Several single institution studies and three meta-analyses had shown the importance of DWI with ADC as a predictor of recurrence and survival in patients with cervical cancer [6,17,18,20,23,36,37]. However, further prospective studies are needed to implement in routine clinical practice.
This report highlights that MRI derived imaging parameters can be a promising and meaningful biomarker of clinicopathological features and prognosis. However, the cost and logistics of MRI imaging is an important factor in routine clinical implementation. In developing countries like India, where carcinoma cervix is associated with poor socioeconomic status, affordability and logistics of routine pre-treatment MRI, is an issue that limits the wider application in general and should be considered currently as investigational.
To the best of our knowledge, limited data is currently available from India regarding the prognostic significance of ADC in cervical cancer patients [19].
Limitation
We have not used histogram based ADC analysis which is a limitation of our study.
Conclusion
In conclusion, conventional T2W MRI along with DWI can increase the diagnostic accuracy of evaluation in carcinoma cervix. ADC could be a useful imaging biomarker that can be used as a surrogate for clinicopathological prognostication. Intensification of treatment regimen based on ADC value and the prognostic significance of absolute change in ADC value after treatment requires further research in future. Whether, ADC can be used as a surrogate marker for tumour microenvironment also needs further evaluation.