Introduction
Ventilator Associated Pneumonia (VAP) is the second most common infection with high mortality (24-50%). Ondansetron is a reliable and safe drug and it is currently used in the prevention of nausea and vomiting and has no side effects.
Aim
The aim of the present study was to examine the effect of ondansetron on prevention of VAP in Intensive Care Unit (ICU) patients.
Materials and Methods
The present study was a randomized clinical trial study (IRCT201406156480N6), carried out at Kashani Hospital, Iran, in 2013 on 80 patients aged from 15-65 years. The patients were randomly allocated to two groups: Case group (n=40) and Control group (n=40). The patients in first group were injected with 4 mg ondansetron, twice daily for five days. The patients of other group were injected with distilled water as placebo. The presence of VAP was assessed in the two groups. The collected data were analysed by SPSS software through Fisher-exact test.
Results
Eleven (13.8%) patients were diagnosed with VAP. Among them, 9 (81.8%) patients were male and 2 (18.2%) patients were female. The incidence of VAP in Case group was 5 (12.5%) patients and in Control group was 6 (15%) patients (p>0.05). Results showed that VAP in Case group was less prevalent than that in the Control group, but this difference was not significant.
Conclusion
The study did not find an association between ondansetron administration and reduction in VAP incidence; vomiting alone may not be leading to VAP, instead silent micro aspirations may be the cause of it. None of the factor such as age, sex, weight, smoking, drug addiction was found significantly related to VAP. Only variable found related was comorbidity.
Introduction
Hospital Associated Pneumonia (HAP) is the parenchymal infection of the lung that is not present while referring to the hospital and start after a minimum of 48 hours of hospitalization [1]. The incidence of HAP is between 30-60% and it is the second most common infection after urinary tract infections in the United States of America [2].
While, VAP is a subset of HAP including all patients supported by mechanical ventilation at the time of infection, VAP occurs almost exclusively in the ICU and represents about 86% of all HAP occurring in ICU [3].
Most of the patients are under mechanical ventilation only for a short time period and half of all the cases of VAP occur during the first four days after placement of an endotracheal tube. The risk of VAP is highest, early in the course of hospital stay, and is estimated to be 3% per day during the first 5 days of ventilation, 2% per day during days 5 to 10 of ventilation, and 1% per day after that [4].
Mortality due to nosocomial pneumonia is around 24% to 50% and if the infection is associated with other risk factors, its mortality is increased to 76% [3,5]. Its highest occurrence is observed in patients with bacteraemia, patients infected with high-risk pathogens (e.g., Pseudomonas aeruginosa) and occur in patients admitted to the ICU [4].
A study carried out by Yavagal DR et al., investigated the role of the metoclopramide in the prevention of HAP, in 305 ICU patients who received enteral nutrition, it was concluded that metoclopramide does not have any role in reducing HAP [6].
In another study, Paul AK et al., studied the effects of metoclopramide and ranitidine in the prevention of aspiration, it was shown that metoclopramide and ranitidine combination regimen was much better and more effective than either of them alone to reduce the risk factors associated with aspiration of gastric contents [7].
A study carried out by Ranjbar H et al., on 80 patients admitted to the ICU, evaluated the effect of chlorhexidine gluconate mouthwash in the prevention of VAP. Selective rinsing of the oral cavity with chlorhexidine gluconate mouthwash, twice a day did not show any significant difference in preventing VAP as compared to normal saline solution, but it can reduce the incidence of late-onset pneumonia and is effective in patients with more severe condition [8].
In a study carried out by van der Maarel-Wierink CD et al., in elderly and disabled patients, it was confirmed that oral hygiene including toothbrushing after every meal and oral cleaning every day and professional health care every week is the best way to reduce aspiration pneumonia [9].
A study by Kelly SD investigated the usefulness of subglottic secretion drainage technology as a method of preventing VAP, it was suggested that subglottic secretion drainage is a cost-effective preventive method [10].
A study by Shiohara Y et al., in Japan checked the influence of Angiotensin Converting Enzyme (ACE) inhibitor drugs in preventing pneumonia in patients with a history of stroke, the results of meta-analysis of five studies on 8693 Asian patients was examined. It was concluded that ACE inhibitor drugs, in comparison with other anti-hypertensive drugs or placebo were more effective in reducing the risk of pneumonia in patients with history of stroke [11].
In a study by Cohen IT et al., the acceptability and efficacy of ondansetron was assessed, the results of the study showed that the incidence of vomiting was significantly less in the ondansetron group [12]. In a study by Salvucci AA et al., the effect of ondansetron in the treatment of nausea and vomiting was examined and it was shown to be safe and an effective drug in the treatment of nausea and vomiting [13].
The main cause of VAP is considered to be, regurgitation of digestive secretions into trachea; therefore, we decided to do this research with the aim of probable reduction in the incidence of VAP. In this study, the effect of ondansetron on prevention of VAP in ICU hospitalized patients was examined.
Materials and Methods
The present study was a randomized clinical trial, with IRCT code: 201406156480N6, carried out in Kashani hospital from September 21st 2012 to December 20th, 2013 on patients aged between 15 and 65 years. Ethics Committee of Shahrekord University of Medical Sciences approved the study. The participants provided the written consent in their native language (Persian) prior to the study. The patients were randomly divided and allocated equally into two groups i.e., Case and Control groups (n=40).
Inclusion criteria: Patients admitted to ICU and on mechanical ventilation, aged between 15 and 65 years, hospitalised for more than 48 hours in ICU and have consented to participate in the study.
Exclusion criteria: Those patients who had suspected or were proved to have lung disease prior to mechanical ventilation were not included in the study.
Case group patients were injected with 4 mg ondansetron (2 ml) twice daily for five days and Control group patients were injected with distilled water as placebo, every 12 hours for five days [14]. The presence of VAP was assessed in two groups based on the clinical, laboratory and radiologic criteria. The injection was administered by a specialist nurse. Patients were examined daily for five days by an anesthesiologist and the CDC criteria was used for the diagnosis of pneumonia [15].
Sample size formula:
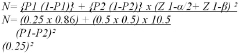
N=39.6 = 40
Patient information was entered in a questionnaire designed for this purpose [Appendix-1].
The collected data were analysed by SPSS software through Fisher exact test.
Diagnoses were done based on CDC criteria defined by Center for Disease prevention and control [15] that included:
For any patient, at least one of the following:
Fever (>38.0°C or >100.4°F)
Leukopenia (≤4000 WBC/mm3) or leukocytosis (>12,000 WBC/mm3)
For adults >70 years old, altered mental status with no other recognized cause.
And at least two of the following:
New onset of purulent sputum or change in character of sputum, or increased
Respiratory secretions, or increased suctioning requirements
New onset or worsening cough, or dyspnoea, or tachypnoea
Rales or bronchial breath sounds
Worsening gas exchange (e.g., O2 desaturations (e.g., PaO2/FiO2 <240)7, increased oxygen requirements, or increased ventilator demand).
Diagnosis was also based on radiographic symptoms: new and persistent or progressive and persistent infiltrate, consolidation or cavitation (which must be present in atleast two consecutive chest radiographs).
Diagnosis based on microbiological criteria:
At least one of the following:
Organism identified from blood
Organism identified from pleural fluid
Positive quantitative culture from minimally-contaminated LRT specimen {e.g., Broncoalveolar Lavage (BAL) or Protected Specimen Brushing (PSB)}
≥5% BAL-obtained cells contain intracellular bacteria on direct microscopic exam (e.g., Gram’s stain)
Positive quantitative culture of lung tissue [15].
Results
The results of this study showed that 11 (13.8%) of the patients suffered from pneumonia, of which 5 (12.5%) patients were from Case group and 6 (15%) patients were from Control group. The difference was not statistically significant (χ2= 0.105, DF=1, p-value=0.745). Patients aged between 15 and 65 years were included in the study.
In the group that had history of comorbidities, 6 (3.33%) patients were suffering from VAP and in group that had no history of comorbidities, 5 (8.1%)patients were suffering from pneumonia. It showed that there was a significant relationship between comorbidity and VAP (p<0.05) [Table/Fig-1].
The association between demographic characteristics, history of smoking, history of drug addiction, history of comorbidity with pneumonia and without pneumonia.
| Variables | Have Pneumonian (%) | Does not have Pneumonian (%) | p-value |
|---|
| Number | 11(13.8%) | 69(86.2%) | NS |
| Age (Mean±SD) | 50.73±6.50 | 43.45±16.22 | NS |
| Weight | 73.54±15.54 | 85.11±9.40 | NS |
| Sex |
| MaleFemale | 9(15.3%)2(9.5%) | 50(84.7)19(90.5) | NS |
| History of smoking |
| YesNo | 2(10)9(15) | 18(90)51(85.1) | NS |
| History of drug addiction |
| YesNo | 1(7.7)10(14.9) | 12(92.3)57(85.1) | NS |
| History of comorbidity with pneumonia |
| YesNo | 6(3.33) 5(8.1) | 12(66.7)57(91.2) | <0.05 |
Fisher exact test
NS: Not significant, p>0.05
p<0.05 was considered to be significan
A total of 5 (45.4 %) of those who had pneumonia were among those who were hospitalized due to head trauma plus major trauma.
Discussion
Pneumonia is one of the common complication that occurs while using mechanical ventilation devices. This condition causes increased mortality and the economics of VAP include increased ICU length of stay (from 4 to 13 days) and an increased health care cost. Aspiration is the most common cause of bacterial pneumonia, such that the mortality rate in patients with aspiration of gastric contents was reported to be 30-60% [16].
Nowadays, ondansetron as antiemetic drug is taken into consideration, and several studies have examined its effectiveness and had positive view on its quality in controlling vomiting [12]. Since vomiting is one of the risk factors of aspiration, in this study, the role of ondansetron in the prevention of VAP was examined.
In some studies, role of metoclopramide in the prevention of aspiration and hospital pneumonia were studied but no similar research has been done on the direct impact of Ondansetron in the prevention of VAP [6].
Each of the factors i.e., age, sex, weight, smoking history, history of drug addiction, cause of hospitalisation and systemic disease were separately studied as was done in a similar study [7].
Nausea and vomiting may have broad causes which include side effects of medications, systemic disorders or infections, vestibular dysfunction, central nervous system infection or increased pressure, peritonitis, hepatic or biliary disorders, radiation or chemotherapy and gastrointestinal obstruction and motility disorders. Vomiting performance of ondansetron is limited to attributable vagal stimulation (stimulation of the gastrointestinal mucosa) and it poorly controls the other drivers of vomiting.
In a study by Farhat K et al., it was observed that the prophylactic use of ondansetron is more effective than metoclopromide, with lesser side effects, in preventing Post Operative Nausea Vomitimg (PONV) in adult females undergoing laproscopic cholecystectomy [17].
In the study by Chio DK et al., combination of ramosetron and ondansetron was used [18]. In the study by Ekinci, ondansetron in combination with tropitzerone and dopidole was used [19]. So, probably a combination of ondansetron with such medications is effective in reducing PONV. In some studies, combination of antibiotic and sucralfite has also been used for gastric cleaning and prevention of pneumonia aspiration [20,21]. One of the most important difference in the incidence of VAP between these studies was difference in methods to control infection and prevention of VAP in ICU.
Differences in diagnostic methods used in studies play a role in the frequency of VAP. It seems that studies examined incidence of VAP based mostly on clinical methods and reported higher prevalence as compared to studies using laboratory methods, especially PSB and BAL.
None of the factor such as age, gender, smoking or drug addiction did reflect a statistically significant difference. Although, in few studies higher age is reported as one of the factor associated with VAP and increased mortality among patients on mechanical ventilation [22,23]. Also, there is no single point of view about the effects of age on the incidence of pneumonia; some studies have reported, age over 70 years as one of the risk factors for developing hospital pneumonia [24].
Also, there is no single point of view regarding the effect of gender on the incidence of VAP. Some studies have reported males and some other have reported females to be at risk factor for developing VAP [24,25]. The results of the present study showed that there was no significant difference between the two sexes and the incidence of VAP.
The results of the present study showed that smoking history in patients receiving mechanical ventilation was not associated with the incidence of VAP and this result is consistent with those of a study conducted in 2005 in which it was observed that there was no relationship between smoking history and pneumonia associated with ventilators [26].
The results of the present study showed that the incidence of VAP is more common in people with a history of underlying disease or comobidity which is consistent with the results of a study conducted by da Silva JM Jr et al., [27].
The mechanically ventilated patients need Nasogastric Tube (NGT) for feeding, NGT causes incomplete closing of the lower oesophageal sphincter and consequently secretions come up and thus, by silent aspiration may cause VAP. Thus, if we can replace NGT by another method like jejunostomy and conduct the study again, it is likely to achieve new results.
Limitation
In this study, no significant relationship between ondansetron and incidence of VAP was observed. The number of subjects was likely not enough to determine this relationship. Further research is required with different research projects to determine the efficacy of ondansetron on VAP. Also, according to the drug half-life (4 to 9 hours), perhaps if the drug was injected with the higher dose the efficacy would have been more.
Conclusion
Ondansetron alone may have no effect on VAP and vomiting alone does not lead to VAP, instead silent aspiration causes it. None of factors such as age, sex, weight, smoking, drug addiction was shown to be effective in VAP. Instead comorbidity was shown to be influential in VAP.
Fisher exact test
NS: Not significant, p>0.05
p<0.05 was considered to be significan
[1]. Vasylius S, Sipylaite J, Lvaskevicius J, Intensive care acquired infection and impact on morbidity and mortalityActa Anaesthesia Scand 2003 47:1132-37. [Google Scholar]
[2]. Richards MJ, Edwards JR, Culver DH, Gaynes RP, Nosocominal infections in combined medical surgical intensive care units in the United StatesInfect Control Hosp Epidemiol 2000 21:510-15. [Google Scholar]
[3]. Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Incidence of and risk factors for ventilator-associated pneumonia in critically ill patientsAnn Intern Med 1998 129:440 [Google Scholar]
[4]. Nassaji M, Mousavi SH, Ghorbani R, Incidences of nosocomial pneumonia in patients above 15 years in intensive care units of university hospital in SemnanKoomesh 2004 5(1):89-94. [Google Scholar]
[5]. Chastre J, Fagon JY, Ventilator-associated pneumoniaAm J Respir Crit Care Med 2002 165:867-0903. [Google Scholar]
[6]. Yavagal DR, Karnad DR, Oak JL, Metoclopramide for preventing pneumonia in critically ill patients receiving enteral tube feeding: a randomized controlled trialCrit Care Med 2000 28(5):1408-11. [Google Scholar]
[7]. Paul AK, Banerjee B, Effect of metoclopramide and ranitidine on gastric fluid volume and its acidityJ Indian Med Assoc 1990 88(8):220-20. [Google Scholar]
[8]. Ranjbar H, Jafari S, Kamrani F, Alavi Majd H, Yaghmaei F, Asgari A, Effect of Chlorhexidine gluconate oral rinse on late onset ventilator associated pneumonia prevention and its interaction with severity of the illnessIranian Journal of Critical Care Nursing 2010 3(2):81-86. [Google Scholar]
[9]. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C, Oral health care and aspiration pneumonia in frail older people: a systematic literature reviewGerodontology 2013 30(1):3-9. [Google Scholar]
[10]. Kelley SD, Number needed to treat for subglottic secretion drainage technology as a ventilator-associated pneumonia prevention strategyCrit Care 2012 16(5):446 [Google Scholar]
[11]. Shinohara Y, Origasa H, Post-stroke pneumonia prevention by angiotensin-converting enzyme inhibitors: results of a meta-analysis of five studies in AsiansAdv Ther 2012 29(10):900-12. [Google Scholar]
[12]. Cohen IT, Ondansetron oral disintegration tablet: acceptability and efficacy in children undergoing adenotonsillectomyJ Aneth Analg 2005 101(1):59-63. [Google Scholar]
[13]. Salvucci AA, Squire B, Burdick M, Luoto M, Brazzel D, Vaezazizi R, Odansetron is safe and effective for prehospital treatment nausea and vomiting by paramedicsPrehosp Emerg Care 2011 15(1):34-38. [Google Scholar]
[14]. Guidelines for the management of adults with hospital-acquired, ventilator associated, and healthcare-associated pneumoniaAm J Respir Crit Care Med 2005 171:388-416. [Google Scholar]
[15]. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf [Google Scholar]
[16]. Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical centreCrit Care Med 2003 31(5):1312-17. [Google Scholar]
[17]. Farhat K, Pasha AK, Kazi WA, Comparison of Ondansetron and Metoclopramide for PONV Prophylaxis in Laparoscopic CholecystectomyJournal of Anesthesia & Clinical Research 2013 4:297 [Google Scholar]
[18]. Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgeryActa Anaesthesiol Scand 2010 54(8):962-69. [Google Scholar]
[19]. Ekinci O, Malat I, Işıtmangil G, Aydın N, A randomized comparison of droperidol, metoclopramide, tropisetron, and ondansetron for the prevention of postoperative nausea and vomitingGynecol Obstet Invest 2011 71(1):59-65. [Google Scholar]
[20]. Silvestri L, van Saene HKF, de la Cal MA, Sarginson RE, Thomann C, Prevention of ventilator-associated pneumonia by selective decontamination of the digestive tractEur Respir J 2008 32(1):241-43. [Google Scholar]
[21]. Kollef MH, Prevention of hospital-associated pneumonia and ventilator-associated pneumoniaCrit Car Med 2004 32(6):1396-405. [Google Scholar]
[22]. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, Guidelines for Preventing Health-Care--Associated Pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory CommitteeMMWR 2004 53(RR03):1-36. [Google Scholar]
[23]. Myrianthefs PM, Kalafati M, Samara I, Baltopoulos GJ, Nosocomial pneumoniaCrit Care Nurs Q 2004 27(3):241-57. [Google Scholar]
[24]. Stéphan F, Mabrouk N, Decailliot F, Delclaux C, Legrand P, Ventilator-associated pneumonia leading to acute lung injury after trauma: importance of Haemophilus influenzaeAnesthesiology 2006 104(2):235-41. [Google Scholar]
[25]. Emilio B, Ana P, Patricia M, Pérez J, Cristina R, Carlos S, Ventilator-associated pneumonia after heart surgery: A prospective analysis and the value of surveillance 2003 31(7):1964-70. [Google Scholar]
[26]. Noor A, Hussain SF, Risk factors associated whit development of ventilator associated pneumoniaJ Coll Physicians Surg Pak 2005 15(2):92-95. [Google Scholar]
[27]. da Silva JM Jr, Rezende E, Guimarães T, dos Campos EV, Magno LA, Consorti L, Epidemiological and microbiological analysis of ventilator-associated pneumonia patients in a public teaching hospitalBraz J Infect Dis 2007 Oct 11(5):482-88. [Google Scholar]