Prevention of Parent to Child Transmission of HIV: Single Centre Experience of 14 years at Tertiary Care Hospital in Delhi, India
AG Radhika1, Sonia Chawla2, Sruthi Bhaskaran3
1 Senior Specialist, Department of Obstetrics and Gynaecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
2 Senior Resident, Department of Obstetrics and Gynaecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
3 Assistant Professor, Department of Obstetrics and Gynaecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. AG Radhika, Senior Specialist, Department of Obstetrics and Gynaecology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Dilshad Garden, Delhi-110095, India.
E-mail: raradhikaag@gmail.com
Introduction
Prevention of Parent To Child Transmission (PPTCT) of HIV/AIDS is an integral component of AIDS control programme. PPTCT is an ongoing programme since last 15 years.
Aim
The aim of the study was to evaluate the reduction in the burden of disease in newborn and infants by prevention of parents to child transmission of HIV/AIDS.
Materials and Methods
This retrospective study was conducted at Department of Obstetrics and Gynecology in a tertiary care hospital of Delhi from May 2002 to May 2015. The data was collected from records of maternal details registered at PPTCT clinic as well as list of infants undergoing Early Infant Diagnosis (EID) recorded in the standard format as per instructions from National AIDS Control Organization (NACO) of India. The Programme performance was assessed against performance indicators stated by NACO, India.
Results
Evaluation was done by dividing study period into two halves of seven years each. Out of 2,52,447 new antenatal case registration, overall, 43% received pretest of which, 91% were tested. Antenatal seropositivity rate varied from 0.1%-0.25%. Of 243 seropositive antenatal women 187 partners tested positive. While 25 women opted for MTP, 15 had still births. There were 17 neonatal deaths at 3-12 months attributable to respiratory infections and diarrheal diseases. Operative delivery rates declined from 50% to 31% over the years. Most women opted for breast feeding. The lost to follow up rate of newborns was quite high with details of only 43.5% being available at 18 months of infant’s age. A total of three infants tested HIV positive at 18 months of age.
Conclusion
The study highlights the practical aspects of policy implementation and operational issues involved in low resource country.
Antenatal woman, Antiretroviral therapy, Nevirapine
Introduction
PPTCT of HIV/AIDS is an integral component of AIDS control programmes since Mother To Child Transmission (MTCT) of HIV/AIDS accounts for over 90% of new infections in children [1]. Without any intervention, the risk of MTCT is estimated at 15%-45% which is reduced to below 5% with the current strategy [2]. Till the year 2013, single dose evirapine (NVP) to the mother baby pair was practiced in India. This reduced the risk of (MTCT) to 16% (in breastfed) and 11% (with replacement feed) [3]. Concerns of viral resistance with Sd-Nevirapine compromising subsequent treatment led to the change in strategy. Based on the current policy of NACO, India, is to prescribe lifelong Antiretroviral Therapy (ART) to all seropositive pregnant women regardless of CD4 count or WHO clinical stage, both for their own health and to prevent vertical HIV transmission from mother to child [4]. An estimated 2.1 million PLHIV (People Living with HIV/AIDS) live in India; of these women constitute 39% while 7% are children below 15 years [5]. With such high numbers, PPTCT is of utmost importance to control the number of newborns infected with the virus.
With the introduction of HAART (highly active antiretroviral therapy), HIV transmission has been reduced significantly. As also reported by Ngemu et al., in 2014, viral load in mother was significantly reduced with HAART (Z=11.324, p<0.001) and about 90% of the children were HIV negative [6].
In another study, in an urban hospital in Angola (2012), it was reported that estimated rates of HIV transmission and death in infants was 8.5% if mother is on ART and 38.9% without ART on a median follow up of 74 weeks [7].
Guru Teg Bahadur (GTB) Hospital caters to the highly populous East Delhi and West Uttar Pradesh belt. The PPTCT programme started at the hospital in 2002 but ART service were established in November 2005.
The PPTCT strategy which is carried out at the Antenatal Clinic of the hospital includes group counselling followed by individual consent. “Opt out” approach is adopted while offering HIV testing. After obtaining an informed consent, HIV testing is done using Rapid test kits namely, Comb-aid (SD diagnostics Ltd.), AIDS-scan trispot (Bharat biotechnology Ltd) and SD Bioline. These kits are supplied by NACO. According to NACO policy, all three tests are required to be positive to diagnose seropositivity [8]. The report is available within 24 hours of testing. Post-test counselling was done for all the women who reported for collecting reports of HIV test before revealing the result. Pregnant women testing positive for HIV are advised Pap smear & VDRL testing, referral to ART centre, TB centre and importance of institutional delivery is re-emphasised. These women were also informed about the neonatal feeding options, neonatal follow up with Dried Blood Smear (DBS) and neonatal Antiretroviral (ARV) prophylaxis. Information about the benefits of use of condom, partner testing is also imparted. Women requesting for pregnancy termination were referred to the Family Welfare clinic for appropriate management.
The aim of the study was to study the impact of PPTCT programme in reduction disease burden in newborn and infants.
Materials and Methods
The PPTCT programme started in the year 2002 at GTB Hospital. The staffs posted at the centre are two counsellors and laboratory technicians each. This retrospective study was conducted at a tertiary care teaching hospital of Delhi, India. The Institutional ethical clearance was obtained before commencement of the study. Data of 14 years from May 2002 to May 2015 was extracted for retrospective analysis from hospital records. Other than the demographic details, the following performance indicators were assessed (as per NACO Guideline) [9]:
Number of New ANC (Antenatal Clinic) Registrations;
Number of pregnant women provided with pre-test counselling;
Out of all registered number of pregnant women tested for HIV;
Total number of pregnant women detected HIV positive;
Number of pregnant women who received post-test counselling and given test results;
Number of spouses/partners of HIV infected pregnant women tested for HIV;
Number of spouses/partners of HIV infected pregnant women found infected;
Number of HIV infected pregnant women who underwent MTP (Medical termination of pregnancy);
Total number of deliveries of HIV infected women (vaginal + LSCS);
Total number of HIV infected women opting for breast feed for first six months;
Total number of HIV infected women opting for replacement feeding for first six months
Treatment of HIV positive pregnant women was based on the existing NACO recommendations. The women received combination of Ziduvudine, Nevirapine and Lamivudine for CD4 count less than 350/ml3 or peripartum single dose nevirapine to mother baby pair when CD4 counts were more than 350/ml3 till a change of policy resulting in Tenovofir, Lamivudine and Efavirenz to all positive women with prolonged coverage of the neonate 2013 onwards. Of the total, 218 mother baby pairs (89.7%) received prophylactic ART coverage.
Statistical Anaylsis
Statistical evaluation of the results was done by using Computer Software SPSS Version 20.0.
The results are expressed in percentage.
Results
The results were viewed separately in two blocks of seven years each i.e., 2002-08 and 2009-15 since there was an obvious difference in performance. Teething problems of a new programme compounded by deficient manpower and inadequate data maintenance were evident in the initial seven years. Compilation of data in the period 2002 to 2009 was found difficult due to non uniform recording. Total of 2,52,447 new antenatal registrations were recorded in the period of 14 years from 2002 to 2015. Overall, 43% (28.2% and 54.6%) of antenatal mothers received pretest counseling of which, 91% were tested [Table/Fig-1] Antenatal seropositivity rate varied from 0.09%-0.25% [Table/Fig-2]. Highest rate of seropositivity was 0.25%, reported in year 2015 and least rate was 0.09% in the year 2007 [Table/Fig-2].
PPTCT services in Antenatal clinic (2002-2015).
Year wise prevalence trend of HIV positive patients.
| year | Prevalence (%) |
|---|
| 2002 | 0.24 |
| 2003 | 0.15 |
| 2004 | 0.16 |
| 2005 | 0.13 |
| 2006 | 0.23 |
| 2007 | 0.09 |
| 2008 | 0.24 |
| 2009 | 0.12 |
| 2010 | 0.18 |
| 2011 | 0.13 |
| 2012 | 0.21 |
| 2013 | 0.18 |
| 2014 | 0.20 |
| 2015 | 0.25 |
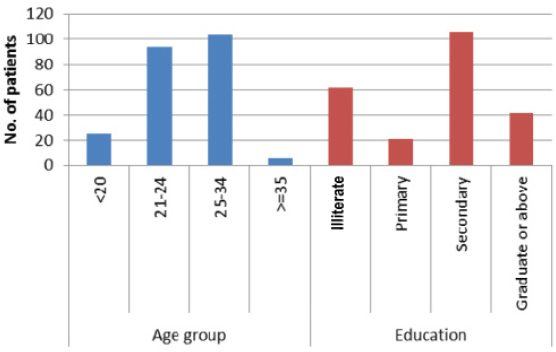
Heterosexual route was the most common mode for disease acquisition. Demographic characteristics are shown in [Table/Fig-3] Despite efforts, testing of spouse was limited to seropositive women. 187 partners tested positive of which 41% were migrant workers, 21% drivers, 1.6% were intravenous drug users.
Demographic characters of seropositive patients.
Overall, 243 seropositive antenatal women were detected in the 14 year period. While 25 women opted for MTP, 15 had still births. Of the remaining 203 sero-positive pregnant women, there were 17 neonatal deaths at 3-12 months attributable to respiratory infections and diarrheal diseases. Seven of these infants were positive by DBS at three months. The lost to follow up rate of infants was very high with details of 43.5% being available at completion of 18 months of infant’s age and of these three babies tested HIV positive. of the total 218 mother who carried on their pregnancies, all the mother baby pairs received prophylactic ART coverage.
Comparing the two blocks of seven years each, there is a definite improvement in each aspect of service delivery as can be observed from [Table/Fig-4]. While the cesarean delivery rates were high at 50% initially, it decreased to 31.4% in the latter half. Overall top feeding was the preferred option (119/186) indicated by the pregnant mother in antenatal clinic. There was improvement in neonatal follow up with reduction in lost to follow up from 70% to 50%.
PPTCT services at our Hospital in two blocks of seven years each.
| 2002-2008 | 2009-2015 | Total |
|---|
| Total number of new ANC | 112,531 | 139,916 | 2,52,447 |
| Pretest counseling | 31,846(28.2%) | 76,509(54.6%) | 108,355(42.9%) |
| HIV Testing | 22,513(70.6%) | 75,643(98.8%) | 98,156(90.2%) |
| Post-test counseling | 20,851(65.4%) | 70,434(93.1%) | 91,285(84.2%) |
| Husbands | 56 | 131 | 187 |
| HIV tests positive | 100 | 143 | 243 |
| MTP | 13 | 12 | 25 |
| Net HIV positive pregnancies | 87 | 131 | 218 |
| Cesarean | 50 | 45 | 95 |
| Normal vaginal delivery | 37 | 86 | 123 |
| Breast feed | 15 | 53 | 68/186* |
| Top feed | 41 | 78 | 119/186* |
| Baby outcome |
| Still births | 9 | 6 | 15 |
| Neonatal deaths | 12 | 5 | 17 |
| Lost to follow up | 46/66 | 59/120 | 105/186 |
| Babies(n=81) on follow-up, 50 > 18 months; 31< 18months |
| Positive at 18 months | 1 | 2 | 3 |
Data of 32 mothers not available.
Discussion
In the current study, there was a definite improvement of services over the 14 years: pretest counseling improved from 28.2% (2002-08) to 54.6% (2009-15), HIV testing from 70.6% to 98.8%. Post-test counseling also improved from 65.4% to 93.1%. Sustained efforts through awareness building activities in antenatal clinic and also among medical and paramedical workers at the hospital, increase in manpower, regular training of counselors could bring this change.
With the use of HAART strategies, PPTCT has been virtually eliminated in developed countries [10]. However, the prevention of transmission has still remained a major challenge for developing countries. Globally, an estimated 3,70,000 children were newly infected with HIV in 2009, and most of them were from low and middle income countries [11]. The HIV prevalence observed among ANC clinic attendees is considered a proxy for HIV prevalence in general population. Its prevalence in 2014-15 was 0.29% (90% CI: 0.28-0.31) and 0.25% in Delhi [5]. Average seroprevalence rate seen in our study was also 0.25%.
The National Agency for Control of AIDS, Nigeria in 2010 reported that only 16.9% of women were tested for HIV [12]. According to UN general assembly special session report, just 20% of the annual estimates of pregnant women in India were counseled and tested for HIV in 2009. Furthermore, only 30% of the estimated HIV infected pregnant women were identified; of these about 60% received sd-NVP which was the national protocol at that time [13]. Antenatal coverage of PPTCT service has been reported to range from 52.4% [14] to more than 95% [15,16]. The opt out approach has consistently resulted in >95% uptake of HIV antibody testing across a variety of international settings [17]. Indian studies have reported testing rates ranging from as low as 64.3% [18] to as high as 92.6% [15]. The percentage of pregnant women accepting HIV test (as percentage of pre-test counseled women) in all the SACS was found to be about or over 90% during 2007-2008 [19]. Testing rates improved from 70.6% in earlier years to 98.8% in the present study too.
Among all the seropositive women delivered at our hospital, average cesarean and normal vaginal delivery rate was 43.5% and 56.4% respectively. In the initial years, cesarean rates were quite high (57%) but with continuous trainings the trends changed and in the later years with higher rates of vaginal delivery (65.6%). Vaginal delivery rates of 46%-66% [20,21] have been mentioned in other studies from India. In this era of HAART, with MCTC as low as 0% following vaginal delivery, high rates of operative delivery point towards an urgent need to sensitize the staff [22]. Majority of women in the present study were in the age group 21-34 years (86.4%) and most common route was heterosexual transmission (90.5%). Similar findings have also been observed in other studies [23,24].
A major limitation of our study continues to be high lost to follow up of infants initially upto 70% but later reduced to 50% with the introduction of dried blood spot at six weeks. Other studies from India have also reported quite high lost to follow up rates ranging from 29% to 67.8% [17,25,26] while in African studies it is seen to vary from 20.8% to 66% [27-29]. Reason for such high rates could be due to high migration rates, birth of a sibling resulting in shift of focus from affected child and to a lesser extent due to lack of awareness despite the high intensity awareness building strategies. These findings emphasize the need for actively developing a tracking system. Operational research studies have evaluated different healthcare delivery models for providing PMTCT services. In a study from western Kenya outcome was compared between 179 HIV-exposed infants seen at clinics that integrated PMTCT follow up services into Maternal and Child Health (MCH) services and 184 infants in an HIV comprehensive care clinic from age six weeks to 12 months [30]. Follow up was better through PMTCT services that were integrated into the MCH clinics compared to delivery of services in the HIV comprehensive care clinics. Seropositivity among the infants could not be calculated in the current study due to high rate of lost to follow ups. Various studies in India have reported seropositivity rate ranging from 3.6% to 15% [15,20].
Involvement of male partner is an essential component for successful implementation of PPTCT programme. In the initial years of our programme, couple counselling and testing was done but since majority of the time the husbands were not found to be accompanying the women, their participation remained low. Hence, testing was insisted for the husbands of serospositive women only. Most of the partners (87.7%) were found HIV positive. Similar findings were also observed by Chauhan G et al. [31].
Despite an improvement over the years, the lost to follow up rates of infants remains high.
Conclusion
This report highlights the strengths and weaknesses thereby of the PPTCT programme developed over 14 years thereby providing an opportunity for improvement. It also gives a perspective of the practical aspects of policy implementation and operational issues involved in low resource country.
*Data of 32 mothers not available.
[1]. Chukwuemeka IK, Fatima MI, Ovavi ZK, Olukayode O, The impact of a HIV prevention of mother to child transmission program in a Nigerian early infant diagnosis centreNiger Med J 2014 55(3):204-08. [Google Scholar]
[2]. World Health Organization (WHO)Mother to Child transmission of HIV 2010 [Google Scholar]
[3]. Nataraj S, Ramanathan M, The Prevention of Parent-to-child Transmission Programme: Is it fair to women?Indian J Med Ethics 2014 11(2):71-75. [Google Scholar]
[4]. Consolidated Guidelines On The Use Of Antiretroviral Drugs For Treating And Preventing HIV Infection Recommendations For A Public Health Approach JUNE 2013WHOAccessed September 5 2016Available online at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 [Google Scholar]
[5]. HIV Sentinel Surveillance Report, A Technical brief 2014-15Accessed September 5 2016Available online at: http://www.naco.gov.in/upload/2016%20Data/SIMU/HIV_Sentinel_Surveillance_report.pdf [Google Scholar]
[6]. Ngemu EK, Khayeka-Wandabwa C, Kweka EJ, Choge JK, Anino E, Oyoo-Okoth E, Effectiveness of option B highly active antiretroviral therapy (HAART) prevention of mother to child transmission (PMTCT) in pregnant HIV womenBMC Res Notes 2014 7:52 [Google Scholar]
[7]. Lussiana C, Clemente SV, Ghelardi A, Lonardi M, Pulido Tarquino IA, Effectiveness of a prevention of mother-to-child HIV transmission programme in an urban hospital in AngolaPLoS One 2012 7(4):e36381 [Google Scholar]
[8]. National AIDS Control Organisaiton, Ministry of Health & Family Welfare, Government of India. National Guidelines for HIV testing 2015 Accessed on 30th May 2017Available from: http://naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Testing_21.Apr2016.pdf [Google Scholar]
[9]. National AIDS Control Programme – Phase IIICore indicators for monitoring & evaluationAccessed on 30th May 2017Available from: http://www.naco.gov.in/sites/default/files/Core%20Indicators%20for%20Monitoring%20&%20Evaluation.pdf [Google Scholar]
[10]. Darak S, Panditrao M, Parchure R, Kulkarni V, Kulkarni S, Janssen F, Systematic review of public health research on prevention of mother-to-child transmission of HIV in India with focus on provision and utilization of cascade of PMTCT servicesBMC Public Health 2012 12:320 [Google Scholar]
[11]. UNAIDS: Report on global AIDS epidemic 2010 [Google Scholar]
[12]. Iwelunmor J, Ezeanolue EE, Airhihenbuwa CO, Obiefune MC, Ezeanolue CO, Ogedegbe GG, Sociocultural factors influencing the prevention of mother-to-child transmission of HIV in Nigeria: a synthesis of the literatureBMC Public Health 2014 14:771 [Google Scholar]
[13]. UNGASS: India: country progress report NACO, Ministry of Health and Family Welfare, Government of India 2010 [Google Scholar]
[14]. Moth IA, Ayayo AB, Kaseje DO, Assessment of utilisation of PMTCT services at Nyanza Provincial Hospital, KenyaSahara J 2005 2(2):244-50. [Google Scholar]
[15]. Mohite RV, Mohite VR, Performance of the prevention of parent to child transmission program: A decadal trend from rural Maharashtra, IndiaIndian J Sex Transm Dis 2016 37(1):52-57. [Google Scholar]
[16]. Chaudhuri S, Mundle M, Konar H, Das C, Talukdar A, Ghosh US, Utilization of therapeutic intervention to prevent mother to child transmission of HIV in a teaching hospital in Kolkata, IndiaJ Obstet Gynaecol Res 2010 36(3):619-25. [Google Scholar]
[17]. Mukherjee S, Ghosh S, Goswami DN, Samanta A, Performance evaluation of PPTCT (Prevention of parent to child transmission of HIV) programme: an experience from West BengalIndian J Med Res 2012 136(6):1011-19. [Google Scholar]
[18]. Baggaley R, Hensen B, Ajose O, Grabbe KL, Wong VJ, Schilsky A, From caution to urgency: the evolution of HIV testing and counselling in AfricaBull World Health Organ 2012 90:652 [Google Scholar]
[19]. Evaluation of the GFATM Round II project ‘Scaling up Prevention of Parent to Child Transmission of HIV and Antiretroviral Treatment, involving public private sector’ Final report 2010Accessed September 5 2016Available online at: http://www.naco.gov.in/NACO/Quick_Links/Publication/Basic_Services/Others/Mid_Term_Evaluation_of_the_GFATM_Round_2_Project_Scaling_up_prevention_of_parent_to_child_transmission_of_HIV_and_antiretroviral_treatment_involving_public_private_sector/ [Google Scholar]
[20]. Joshi U, Kadri A, Bhojiya S, Prevention of parent to child transmission services and interventions - coverage and utilization: A cohort analysis in Gujarat, IndiaIndian J Sex Transm Dis 2010 31(2):92-98. [Google Scholar]
[21]. Shah SJ, Trivedi YN, Morjaria K, Incidence of HIV in low risk population - antenatal women and rate of vertical transmissionGujarat Med J 2015 70:29-31. [Google Scholar]
[22]. Boer K, Nellen JF, Patel D, Timmermans S, Tempelman C, Wibaut M, The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal deliveryBJOG 2007 114(2):148-55. [Google Scholar]
[23]. Gupta S, Gupta R, Singh S, Seroprevalence of HIV in pregnant women in North India: a tertiary care hospital based studyBMC Infect Dis 2007 7:133 [Google Scholar]
[24]. Baig VN, Swarnkar M, Bhardwaj AK, Rathore M, Kasyap A, A study on sociodemographic profile and risk factors present in HIV infected patients attending art centre in tertiary care hospital in Rajasthan, IndiaNat J Commun Med 2012 3:339-43. [Google Scholar]
[25]. Panditrao M, Darak S, Kulkarni V, Kulkarni S, Parchure R, Sociodemographic factors associated with loss to follow-up of HIV-infected women attending a private sector PMTCT program in Maharashtra, IndiaAIDS Care 2011 23:593-600. [Google Scholar]
[26]. Goswami S, Chakravorty PS, Prevention of parent to child transmission of HIV (PPTCT):an effort of 4 years in a tertiary centreJ Obstet Gynecol India 2011 61:394-98. [Google Scholar]
[27]. Ahoua L, Ayikoru H, Gnauck K, Odaru G, Odar E, Ondoa–Onama C, Evaluation of a 5-year programme to prevent mother-to-child transmission of HIV infection in Northern UgandaJ Trop Pediatr 2010 56:43-52. [Google Scholar]
[28]. Oladokun RE, Awolude O, Brown BJ, Adesina O, Oladokun A, Roberts A, Service uptake and performance of the prevention of mother-to-child transmission (PMTCT) programme in Ibadan, NigeriaAfr J Med Med Sci 2010 39:81-87. [Google Scholar]
[29]. Hassan AS, Sakwa EM, Nabwera HM, Taegtmeyer MM, Kimutai RM, Sanders EJ, Dynamics and constraints of early infant diagnosis of HIV infection in rural KenyaAIDS Behav 2012 16:05-12. [Google Scholar]
[30]. Ong’ech JO, Hoffman HJ, Kose J, Audo M, Matu L, Savosnick P, Provision of services and care for HIV-exposed infants: a comparison of maternal and child health clinic and HIV comprehensive care clinic modelsJ Acquir Immune Defic Syndr 2012 61(1):83-89. [Google Scholar]
[31]. Chauhan G, Verma A, Bansal RK, Prasad R, Socio demographic and clinical profile of HIV positive antenatal women registered in PPTCT centres, Surat, GujaratNat J Commun Med 2014 5:337-41. [Google Scholar]