Introduction
The normal vaginal flora is highly complex, dominated by lactobacilli of doderlein that plays a vital role in maintaining the women’s health and inhibits other pathogenic microorganisms. Fluctuation in local environment or exposure to any exogenous and endogenous sources changes the vaginal flora over a period of time. Disruption of the vaginal ecosystem changes the microflora of the healthy vagina, altering the pH and predisposing to lower reproductive tract infections. The change in the microflora of the female genital tract by pathogenic organisms may ascend from vagina to upper genital tract and may cause infertility. Although several studies demonstrate a higher prevalence of bacterial vaginosis in infertile population. The role of vaginal microbiome in infertility is not clear and need to be explored further.
Aim
To compare the vaginal flora and analyse the incidence of asymptomatic vaginosis among healthy women and in women with infertility problems.
Materials and Methods
A cross-sectional study was conducted over a period of six months at Sri Lakshmi Narayana Medical College and Hospital Puducherry, India. A total of 200 high vaginal swabs were collected from Group 1 which included 84 healthy women with regular menstrual cycles without any gynaecological disorder and from Group 2, 116 women with infertility problems attending fertility clinic within the age group of 18 to 45 years. All swabs were subjected to routine aerobic, anaerobic and fungal culture. Saline wet mount was performed for the detection of clue cells and Trichomonas vaginalis, 10% KOH was performed for demonstration of budding yeast cells and pseudo hyphae, Gram’s staining to determine the presence of yeast cells, leucocytes and bacterial morphotypes. The smear was also graded using Nugent scoring system.
Results
The vaginal flora of Group 1 was dominated by Lactobacillus (40, 27.8 %) followed by Micrococcus (22, 15.3 %), Enterococcus (16, 11.1%), Coagulase negative Staphylococcus spp. (12, 8.3%). Whereas in Group 2, the most dominant flora was Candida spp. (30, 26.5 %), Enterococcus (26, 23%) followed by Gram negative bacilli such as E. coli (16, 14.1 %). The percentage of Lactobacillus in Group 2 women with infertility problems was relatively low (4, 3.5%). Asymptomatic vaginosis was present in 32 (27.6 %) of Group 2 women compared to Group 1 women were only 6 (7.1%) had asymptomatic vaginosis.
Conclusion
Women with infertility problems showed higher prevalence of asymptomatic vaginosis and abundance of Bacterial Vaginosis (BV) associated bacteria compared to healthy women. Hence, this study recommends the screening of vaginal flora as a routine for all women, especially in women undergoing infertility treatment and also suggests the importance of vaginal culture and sensitivity in routine practice.
Introduction
The vaginal ecosystem is highly complex, normally colonized with mixed aerobic and anaerobic bacteria that play a major role in women’s health [1-3]. Doderlein bacilli are non-spore forming, Gram-positive rods that are normal inhabitants of female genitourinary tract accounting for 95% and maintaining the vaginal pH 3.5-4.5 [4,5]. Peptococcus spp., Bacteroides spp., Staphylococcus spp., Corynebacterium spp., Peptostreptococcus spp. and Eubacterium spp. are the other microbes that colonize the vagina [6]. Adams M, stated that lactobacilli are protective organisms that inhibit the growth of pathogenic organisms by producing lactic acid and other metabolites [7]. Kirmani N found that lactobacilli are the dominant microflora of the vaginal ecosystem [8]. The ovarian hormones play a vital role in maintaining the normal Vaginal Flora (VF) during the sexually mature period of a women’s life [8]. Forsum U et al., observed that the oestrogen level is believed to change during every menstrual cycle and the recovery of the Lactobacillus varies slightly [9]. Rakoff AE et al., stated, the oestrogen level seems to be a determining factor for colonization of lactobacilli although there is still no convincing data [10]. The relationship between vaginal microbial flora, menstruation and levels of oestrogen is complex [11]. The composition of the vaginal ecosystem is not static, exposure to any endogenous and exogenous influences such as antibiotics, vaginal medications, systemic hormones, contraceptive preparations, douches, frequency of sexual intercourse, vaginal deodorants, stress levels and poor socio-economic status causes fluctuation in local environment and heighten or diminish specific vaginal microbes or alters the VF over a period of time [12]. According to Hay PE et al., BV occurs when lactobacilli are replaced by overgrowth of few anaerobes or vaginal commensals or aerobic organisms, predominantly enteric commensals [13]. Disruption in the normal vaginal ecosystem changes the microflora of the healthy vagina, altering the pH and predisposing it to lower reproductive tract infections such as vaginitis [14]. The changes in the microflora of the female genital tract by pathogenic organisms may ascend from vagina to upper genital tract and may cause infertility [15-17]. Bartlett JG et al., stated that at the onset of puberty changes in the VF especially growth of Lactobacillus and Streptococcus occurs due to the increase in glycogen content [6]. The vaginal secretion has pronounced bactericidal action and this remains during the fertile years and changes during menopause [6,12]. Pabich WL et al., found out in most postmenopausal women, lactobacilli are replaced by uropathogens [18]. Slotnick IJ in 1963 observed that Doderlein’s bacilli is accompanied by streptococci, diptheroids and yeast which prevents the establishment of other possibly harmful microorganisms and constitutes a defense mechanism in the vagina [8,19]. BV can be polymicrobial leading to a common outcome, the area of controversy is mainly due to differences in the development of symptoms among individual women and several studies have concluded that it’s very difficult to correlate VF and BV symptoms [20-23]. Several studies have concluded that clinical criteria cannot accurately predict specific VF alterations mainly due to differences in the development of signs or symptoms among individual women [21-23]. Approximately 30%-35% of the women with problems of infertility are affected with post inflammatory changes of oviduct or surrounding peritoneum that interfere with tubo-ovarian function [24,25] and salpingitis occurs in 15% of women in which 2.5% of women become infertile and all these results from altered VF [25,26]. The association between vaginal microbiome and in vitro fertilization outcome is also warranted, which in turn affects pregnancy outcome [27]. The role of vaginal microbiome in infertility is not clear, although pathogens such as Mycoplasma, Chlamydiatrachomatis, Neisseria gonorrhoeae can cause infertility [27]. Hence, the present study aimed to compare the VF and analyse the incidence of asymptomatic vaginosis among healthy women and in women with infertility problems.
Materials and Methods
This study was conducted at the Department of Microbiology, Sri Lakshmi Narayana Medical College and Hospital, Puducherry, over a period of seven months from 1st June 2015 to 31st Dec 2015.
Inclusion Criteria: All non-pregnant women of reproductive age group of 18-45 years were included. The study participants were categorized in to Group 1 and Group 2. Group 1 includes control group of 84 healthy women with regular menstrual cycles and without any gynaecological disorder. In Group 2, 116 women with infertility problems attending the infertility clinic with either primary infertility or secondary infertility were included.
Exclusion Criteria: Women with chronic autoimmune or inflammatory condition or on any antimicrobials (oral or topical) within the previous four weeks, women using any intrauterine device or hormonal contraceptives and with complaints of discharge, itching, burning and dysuria were excluded from the study.
A total of 200 (Group 1 and Group 2) women who agreed to participate and fullfilled the inclusion criteria were enrolled for the study. The approval for this study was obtained from Institutional Ethical committee and informed consent was obtained from all the participants included in the study. A pre-structured questionnaire was designed to collect the demographic profile from the study population that includes nutritional status, occupational status, educational qualification and socioeconomic profile. Gynaecological examination, including per speculum and per vaginal examinations were performed in all participants using a non-lubricated speculum. Two High Vaginal Swabs (HVS) were collected from each individual with a sterile swab stick from the posterior vaginal fornix and external orifice of uterine cervix after cleaning the vulva with sterile water. One swab was used for 10% KOH mount preparation for examination of yeasts (pseudohyphae or budding yeast cells) and saline wet mount examination for the detection of clue cells (vaginal epithelial cells with heavy coating of bacteria obscuring the peripheral borders) and Trichomonasvaginalis (motile trophozoites with characteristic motility). The second swab was used for smear preparation, culturing of yeast, aerobic bacteria. For the presence of aerobic bacteria, the vaginal swabs were cultured on to 5% sheep blood agar, chocolate agar and MacConkey agar and were incubated at 37°C in a thermostater for 24 hours and prolonged incubation for 48 hours was done if there was no growth after 24 hours and similar standard procedure was followed for anaerobes. For the presence of yeast, the swabs were inoculated on to Sabouraud’s Dextrose Agar (SDA) and incubated at 25±2°C. The colonies were read out as per standard protocol. The growth was identified based on Gram staining, motility, catalase test, oxidase test and other routine biochemical tests like indole, methyl red test, vouges proskauer test, citrate, urease, mannitol motility test, bile esculin hydrolysis, triple sugar iron agar test, coagulase test, etc. Grams staining of the direct smear was performed and interpreted using the Nugent’s scoring system [Table/Fig-1] to determine the composition of bacterial morphotypes, presence of yeasts and leukocytes [Table/Fig-2] [28,29]. This technique relies on the quantification of three different bacterial morphotypes: large Gram-positive bacilli – lactobacilli; small Gram-variable coccobacilli which represent Gardnerella, Bacteroides/Prevotella species and curved rods which represent Mobiluncus species. On the basis of these results, the specimen is assigned a score from 0 to 10, with score 1±3 as normal/healthy VF, 4±6 as intermediate VF and 7±10 as BV/Unhealthy VF [5,16].
Nugent’s scoring system and Interpretation of Nugent score.
| No. of lactobacilli = Score | No. of Gardnerella = Score, | No. of Curved GNB = Score | Sum = *N Score | Interpretation of Nugent score |
|---|
| ≥30 = 0 | 0 =0 | 0 = 0 | 0 | Smear not consistent with BV |
| 5-30 = 1 | <1=1 | <1=1 | 3 |
| 1-4 = 2 | 1-4 =2 | 1-4 =1 | 5 + Clue Cells not present | Smear not consistent with BV |
| 5 + Clue Cells are present | Smear consistent with BV |
| <1 = 3 | 5-30 =3 | 5-30 =2 | 8 | Smear consistent with BV |
| 0 = 4 | > 30 = 4 | > 30 = 2 | 10 |
Laboratory examination of vaginal smears and the determination of the Nugent score / N Score = The sum of the scores for each bacterial morphotype listed below.
Grams stain of high vaginal swab. (showing epithelial cells and numerous Gram positive lactobacilli {purple rods}/100X objective).
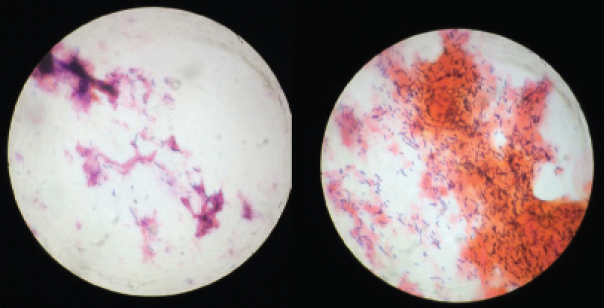
Statistical Analysis
A proportional test was done to find out the significant difference in VF between the two groups. A p-value < 0.05 has been considered as statistically significant.
Results
A total of 200 HVS collected from Group 1 and Group 2 women were screened to analyse the composition of vaginal microbiota and incidence of asymptomatic vaginosis. Out of 84 HVS collected from Group 1 participants i.e., healthy women, 76 showed growth from which 144 isolates were obtained and eight samples did not show any growth. Of the 116 HVS cultured from Group 2 participants i.e., women with infertility problems, 102 samples showed growth from which 113 isolates were obtained and 14 samples did not show any growth [Table/Fig-3]. The total no. of isolates from both Group1 and Group 2 women was 257. No growth was observed in 22/200 (11%) of the individuals studied and overall 178/200 (89%) of HVS showed growth.
Results of high vaginal swab culture.
| Results of culture | HVS from healthy non –pregnant women | HVS from women with infertility problems | Total No. |
|---|
| No. of samples processed | 84 | 116 | 200 |
| No. of samples showed growth | 76 | 102 | 178 |
| No. of samples without growth | 8 | 14 | 22 |
| No. of isolates obtained | 144 | 113 | 257 |
In this study, the VF in both the groups were Lactobacillus, Enterococcus spp, Coagulase Negative Staphylococcus spp., Candida spp., curved Gram negative rods, E. coli and Enterobacter spp. [Table/Fig-4]. The VF in Group1 was dominated with Lactobacillus (40, 27.8%) followed by Micrococcus (22, 15.3%), Enterococci (16, 11.1%), coagulase negative Staphylococcus spp. (12, 8.3%) and Staphylococcus aureus (12, 8.3%) [Table/Fig-4], whereas in Group 2 women with infertility problems the most dominant flora was Candida spp. (30, 26.5%), Enterococcus (26, 23%) followed by coagulase negative Staphylococcus spp. (16, 14.1%), Gram negative bacilli such as E. coli (16, 14.1%) and curved Gram negative rods (8, 7.1%). The percentage of Lactobacillus in Group 2 women was very low (4, 3.5%) compared to Group 1 women. The difference was statistically significant (p=0.023) [Table/Fig-4]. Out of 116 Group 2 women with infertility problems, 96 (82.8 %) had primary infertility and 20 (17.2%) had secondary infertility [Table/Fig-5]. Nugent scoring analysis of the HVS smears and gynaecological examination revealed 32 (27.6 %) women of Group 2 women had asymptomatic vaginosis compared to Group 1 where only 6 (7.1%) women had asymptomatic vaginosis [Table/Fig-6]. Data analysis revealed 28/32 Group 2 women and 5/6 Group 1 women identified with asymptomatic vaginosis were working population whereas only 4/32 Group 2 women and 1/6 Group 1 women who had asymptomatic vaginosis were housewives [Table/Fig-7]. A 3/6 Group 1 women and 13/32 Group 2 women of age group 31 to 35 years showed highest prevalence of asymptomatic vaginosis [Table/Fig-7]. The prevalence of asymptomatic vaginosis was found to be higher in women with education qualification of higher secondary and above [Table/Fig-7].
Comparison of vaginal flora in Group 1 and Group 2 women.
| Organism Isolated | No. of isolates (%) from Group1 Healthy Women n = 144 | No. of isolates (%) from Group 2 women with infertility problems n = 113 | p-value |
|---|
| Enterococcus spp. | 16 (11.1%) | 26 (23%) | 0.032* |
| Micrococcus | 22 (15.3%) | 0% | 0.063 |
| Candida spp. | 10 (6.9%) | 30 (26.5%) | 0.041* |
| Coagulase negative Staphylococcus spp. | 12 (8.3%) | 16 (14.1%) | 0.081 |
| Diphtheroids | 14 (9.7%) | 0% | <0.001* |
| E. Coli | 4 (2.8%) | 16 (14.1%) | 0.071 |
| Staphylococcus aureus | 12 (8.3%) | 0% | <0.001* |
| Pseudomonas spp. | 8 (5.6%) | 0% | <0.001* |
| Enterobacter spp. | 2 (1.4%) | 0% | 0.069 |
| Lactobacillus | 40 (27.8%) | 4 (3.5%) | 0.023* |
| Curved Gram-negative rods | 4 (2.8 %) | 8 (7.1%) | 0.079 |
| Trichomonas Vaginalis | 0 % | 0% | - |
| Group B streptococci | 0% | 8 (7.1%) | 0.068 |
| Gram variable coccobacilli | 0% | 5 (4.4%) | 0.072 |
Note: A proportional test has been done to find out the significant difference in VF between the two groups. A p-value < 0.05 has been considered as statistically significant,
: Statistically Significant.
Prevalence of asymptomatic vaginosis among Group 2 infertile women.
| Study subjects : | No. of cases (%) | No. of cases with asymptomatic vaginosis |
|---|
| Group 2 women with primary infertility | 96 (82.8%) | 30 (93.8%) |
| Group 2 women with Secondary infertility | 20 (17.2%) | 2 (6.3%) |
Prevalence of asymptomatic vaginosis based on gynaecological examination and nugent scoring analysis.
| Observation | Group 1 Healthy women (n = 84) | Group 2 Women with infertility problems (n =116) |
|---|
| Asymptomatic vaginosis | 6 (7.1 %) | 32 (27.6%) |
Analysis of different parameters among Group 1 healthy women and group 2 women with infertility problems.
| Observation/data analysis | No. of Group 1 cases with asymptomatic vaginosis n = 6 | No. of Group 2 cases with asymptomatic vaginosis n = 32 |
|---|
| Working populationHouse wife | 51 | 284 |
| Income Per month in rupeesLess than 50005000 -10,000Above 10,000 | 042 | 4208 |
| Nutritional status:NourishedMalnourished | 51 | 239 |
| Age in years:≤ 2021- 2526 -3031-3536-40≥ 41 | 101301 | 0271346 |
| Educational QualificationIlliteratePrimaryMiddleHigher secondaryGraduate and above | 10122 | 621716 |
Discussion
In present study, the VF of Group 1 women showed 9.7% diphtheroids, 8.3% Staphylococcus aureus, 8.3% coagulase negative Staphylococcus spp., 11.1% enterococci, 15.3% micrococci, 6.9% Candida, 5.6% Pseudomonas, 1.4% Enterobacter, 27.8% lactobacilli, 2.8% E.coli and 2.8% Curved Gram negative rods whereas Caster and jones, found 74% diptheroids, 50% Staphylococcus, 48% anaerobic cocci and bacilli, 38% non-haemolytic streptococci, 30% doderlein’s bacilli, 15% yeast, 90% α-streptococci and 8% E. coli in their study on the VF of normal female [30,31]. Hence, our data suggest that the VF in healthy adult women is a dynamic ecosystem in which lactobacilli was found to be the numerically dominant bacteria. The normal vaginal ecosystem is maintained by lactobacilli that are believed to play a crucial role in producing lactic acid and other substances that inhibit the growth of pathogens and other opportunistic bacteria [1,32].
The present study revealed higher percentage of lactobacilli 27.8 % in Group 1 women compared to Group 2 women who had only 3.5%. Many studies have shown similar results that the number of colonies of lactobacilli was inversely proportional to the number of colonies of Candida spp. in women with fertility problems [8,33,34]. The VF of Group 2 women showed a vast variation/significant difference compared to the resident VF of Group 1 women. These changes could be attributed with the clinical syndrome of BV. Spiegel CA et al., defined BV as disturbance in the VF with large decrease or total loss of lactobacilli and an increase in numbers of facultative anaerobic Gram-negative rods such as E. coli, Gardnerella vaginalis and Mycoplasma [35-37] and epidemiologically BV has been found associated with sexual activity, Sexually Transmitted Diseases (STDs) and douching [38-40].
The Group 1 women had a VF dominated by lactobacilli whereas Group 2 women showed a varied microflora with relatively low lactobacilli and increase in Candida, enterococci, and Gram negative bacilli such as E. coli and curved Gram negative rods was found. The results of our study were in line with the previous reports that have reported that altered VF is more common among infertile women with most dramatic compositional changes in the vaginal microbiota i.e., the depletion of lactobacilli in conjunction with the massive colonization of the vagina with many diverse bacteria [33,41-43] and studies have found the absence or fewer lactobacilli with prevalence of Enterobacteriaceae, Enterococcus spp. and Streptococcus spp. is significantly associated with BV [16]. It was also observed in our study the vaginal microbiota of Group 2 women showed 14.1% of E. coli, however studies have concluded E. coli as a pathogen causing BV; which occurs on the skin of the perineum and genitalia that frequently infects wounds [34].
Nugent’s scoring analysis of HVS smears and gynaecological examination suggested that a significant number 32 (27.6%) of Group 2 women had asymptomatic vaginosis, these results were in conjunction with the earlier studies which showed the incidence of lower reproductive tract infection and BV is more common among infertile women [41,44,45]. In the present study it was also shown that relative to the Group 1 women, the Group 2 women showed higher prevalence of asymptomatic vaginosis and abundance of BV associated bacteria. Recent studies have shown most women with BV are usually asymptomatic and controversies exist as to the appropriate management of these women [41]. Schwebke JR and Desmond R, observed that many women with BV are asymptomatic and remain so for longer period of time [33] with no specific vaginal complaints or rarely present with malodorous discharge [23]. Moreover, the misjudgment of vaginal complaints is rather high and the diagnosis of vaginal infections is not correctly used. This also highlights the fact that lower reproductive tract infection is equally prevalent in asymptomatic infertile women [45,46]. Bang RA et al., found that although 92% of women had gynaecological problems on examination, only 55% of them were symptomatic [47]. Hence, clinical diagnosis and laboratory tests should be emphasized more in the diagnosis of vaginal complaints and regular screening for VF especially in women of reproductive age group is needed for better understanding of the cause, for effective treatment and to prevent infertility problems. Moreover, the VF also varies based on patient groups, geographic settings, etc., therefore more studies on VF are needed or it has to be established before application of these approaches in clinical practice.
In Group 2 women, asymptomatic vaginosis was more prevalent among women with primary infertility compared to those with secondary infertility. Earlier studies have also shown the same that lower reproductive tract infection was higher among women with primary infertility as compared to those with secondary infertility [48]. Though it has not been firmly established what risks infertility, patients with vaginosis from pregnancy outcome hold [8] and it is still not clear whether infertility has led to change in the VF or change in the VF has led to infertility. There could be some possibility of BV hampering the fertility. However, it’s very difficult to correlate or limited data is available on the impact of specific behavior with changes in the VF and attempts made to determine the causes of these fluctuations have been less and have yielded little insight [49]. Another important issue that needs to be addressed is that whether or not to treat women based on the composition of their VF, irrespective of the presence or absence of clinical symptoms. In several studies, it has been found out that BV is strongly associated with reproductive failure, preterm birth as well as other pregnancy complications such as increased risk of late miscarriage [41,44,50]. Hence, there is a need to conduct further studies that could assess various behavioral and socio-demographic factors, predisposing these women to infertility.
Asymptomatic Vaginosis in both the groups was most prevalent in the age group of 31 to 35 years. Studies have shown the highest prevalence of sexually transmitted infection in the age group of 21 to 35 years in our country, which are attributed to higher sexual activities in this age group [51]. Moreover, this is a general phenomenon among the populations of India i.e., lack of hygiene, promiscuity and traditional taboo against openness about these diseases are the usual factors responsible for the high prevalence [45,52,53].
Data analysis showed asymptomatic vaginosis was most prevalent in working population of the study subjects than in house wives. Studies have also documented reproductive tract infections was more common among women working in the fields and rearing domestic animals than in housewives [45,48]. Incidence of asymptomatic vaginosis and its correlation with education has shown higher prevalence of asymptomatic vaginosis was found in women who were educated upto graduate and above. This could be because educated women have more chances of seeking medical help as they are more aware in general. Reason behind this should be investigated and need further research. There are few studies which have correlated infertility to psychosocial stress levels and studies have also proved adverse effect of stress on susceptibility to BV infection [54,55]. Hence, more future research on the mechanisms by which stress contributes to increases in susceptibility to BV is needed. In addition knowledge regarding the fluctuations of the vaginal microbiota due to factors such as menstrual cycle, concomitant infections, other stress, history of contraception and vaginal cleansing habits are required for analysing the significant shifts in the vaginal microbiota and also identification of the VF pattern is essential which helps in better interpretation [34,45,56]. Patients and clinicians may incorrectly interpret the symptoms of vaginosis leading to self-medication or unnecessary prescription. Hence, a more scientific approach is recommended in analysing the vaginal smear and monitoring of women with infertility problems.
Limitation
Biochemical characterization of anaerobes was not done for financial and logistical reasons. Some of the healthy women included in this study also had asymptomatic vaginosis and was not able to correlate with the past history of vaginal infections or STDs due to incomplete recording of data. Moreover, further studies in women from different geographical locations are essential to assess the specific behavioral activities that lead to change in the VF or cause of infertility.
Conclusion
The VF of women with infertility problems showed a relative decrease in lactobacilli, compared to healthy women. Candida and BV associated bacteria were significantly higher in vagina of women with infertility problems. Asymptomatic vaginosis was more common in women with infertility problems compared to healthy women. Moreover, the VF of adult women is a dynamic ecosystem, which changes over a period of time. Hence, this study recommends the screening of VF as a routine for all women, especially in infertile women of reproductive age group and with more future studies in different populations of women that could provide additional information regarding the influence of behavior on the composition of the VF and subsequent risk in the development of infertility.
Laboratory examination of vaginal smears and the determination of the Nugent score / N Score = The sum of the scores for each bacterial morphotype listed below.
Note: A proportional test has been done to find out the significant difference in VF between the two groups. A p-value < 0.05 has been considered as statistically significant,
*: Statistically Significant.
[1]. Donders GG, Definition and classification of abnormal vaginal floraBest Pract Res Clin Obstet Gynaecol 2007 21(3):355-73. [Google Scholar]
[2]. Ma B, Forney LJ, Ravel J, Vaginal microbiome: rethinking health and diseaseAnnu Rev Microbiol 2012 66:371-89. [Google Scholar]
[3]. Hemsell DL, Infections after gynaecologic surgeryObstet Gynaecol Clin North Am 1989 16(2):381-400. [Google Scholar]
[4]. Antonio MA, Hawes SE, Hillier SL, The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these speciesJ Infect Dis 1999 180(6):1950-56. [Google Scholar]
[5]. Redondo Lopez V, Cook RL, Sobel JD, Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microfloraRev Infect Dis 1990 12(5):856-72. [Google Scholar]
[6]. Bartlett JG, Onderdonk AB, Drude E, Goldstein C, Anderka M, Alpert S, Quantitative bacteriology of the vaginal floraJ Infect Dis 1977 136(2):271-77. [Google Scholar]
[7]. Adams M, Safety of industrial lactic acid bacteriaJ Biotechnol 1999 68(2):171-78. [Google Scholar]
[8]. Kirmani N, Normal bacterial flora of vaginaJPMA 1988 38(1):01-03. [Google Scholar]
[9]. Forsum U, Holst E, Larsson PG, Vasquez A, Jakobsson T, Mattsby Baltzer I, Bacterial vaginosis–a microbiological and immunological enigmaAPMIS 2005 113(2):81-90. [Google Scholar]
[10]. Rakoff AE, Feo LG, Goldstein L, The biologic characteristics of the normal vaginaAm J Obstet Gynaecol 1944 47(4):467-94. [Google Scholar]
[11]. Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Influence of the normal menstrual cycle on vaginal tissue, discharge, and microfloraClin Infect Dis 2000 30(6):901-07. [Google Scholar]
[12]. Witkin SS, Linhares IM, Giraldo P, Bacterial flora of the female genital tract: function and immune regulationBest Pract Res Clin Obstet Gynaecol 2007 21(3):347-54. [Google Scholar]
[13]. Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J, Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriageBMJ 1994 308(6924):295-98. [Google Scholar]
[14]. Mahadik KV, Pathak A, Shah H, Purohit MR, Evaluation of vaginal flora and antibiotic prophylaxis in elective hysterectomy in a rural hospital from IndiaInt J Infect Con 2013 9(3) [Google Scholar]
[15]. Casari E, Ferrario A, Morenghi E, Montanelli A, Gardnerella Trichomonas vaginalis Candida Chlamydia trachomatis Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomaticinfertile womenNew Microbiol 2010 33(1):69 [Google Scholar]
[16]. Gupta S, Goyal A, Singh S, Agrawal BM, Outcome of routine microbiological screening for lower genital tract infections in symptomatic non-pregnant females complaining infertilityIOSR-JDMS 2014 13(1):26-29. [Google Scholar]
[17]. Fedele L, Acaia B, Ricciardiello O, Marchini M, Benzi-Cipelli R, Recovery of Chlamydia trachomatis from the endometria of women with unexplained infertilityJ of Reprod Med 1989 34(6):393-96. [Google Scholar]
[18]. Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K, Prevalence and determinants of vaginal flora alterations in postmenopausal womenJ Infect Dis 2003 188(7):1054-58. [Google Scholar]
[19]. Slotnick IJ, Hildebrandt RJ, Prystowsky H, Microbiology of the female genital tract IVcervical and vaginal flora during pregnancy. Obstetrics & Gynaecology 1963 21(3):312-17. [Google Scholar]
[20]. Pépin J, Deslandes S, Giroux G, Sobéla F, Khonde N, Diakité S, The complex vaginal flora of West African women with bacterial vaginosisPLoS One 2011 6(9):e25082 [Google Scholar]
[21]. Linhares IM, Witkin SS, Miranda SD, Fonseca AM, Pinotti JA, Ledger WJ, Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by cultureInfect Dis Obstet Gynaecol 2001 9(4):221-25. [Google Scholar]
[22]. Landers DV, Wiesenfeld HC, Heine RP, Krohn MA, Hillier SL, Predictive value of the clinical diagnosis of lower genital tract infection in womenAm J Obstet Gynaecol 2004 190(4):1004-08. [Google Scholar]
[23]. Schwiertz A, Taras D, Rusch K, Rusch V, Throwing the dice for the diagnosis of vaginal complaints?Ann Clin Microbiol Antimicrob 2006 5(1):4 [Google Scholar]
[24]. Westroem L, Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countriesAm J Obstet Gynaecol 1980 138(7):880-92. [Google Scholar]
[25]. Westrom LV, Sexually transmitted diseases and infertilitySex Transm Dis 1994 21:532-37. [Google Scholar]
[26]. Sweet RL, Diagnosis and treatment of acute salpingitisJ Reprod Med 1977 19(1):21-30. [Google Scholar]
[27]. Hyman RW, Herndon CN, Jiang H, Palm C, Fukushima M, Bernstein D, The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transferJ Assist Reprod Genet 2012 29(2):105-15. [Google Scholar]
[28]. Church D, Miller B, Alberta guideline for laboratory processing and interpretation of vaginal specimens for bacterial vaginosis. Calgary Laboratory Services, Calgary Zone. Alberta Health Services published in ConnectionsFall 2011 3(2) [Google Scholar]
[29]. Nugent RP, Krohn MA, Hillier SL, Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretationJ Clin Microbiol 1991 29(2):297-301. [Google Scholar]
[30]. Caster B, Jones CP, A study of the vaginal flora in the normal femaleSouthern Medical Journal 1937 30(3):298-303. [Google Scholar]
[31]. Lindner JG, Plantema FH, Hoogkamp-Korstanje Ja, Quantitative studies of the vaginal flora of healthy women and of obstetric and gynaecological patientsJ Med Microbiol 1978 11(3):233-41. [Google Scholar]
[32]. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI, Host-bacterial mutualism in the human intestineScience 2005 307(5717):1915-20. [Google Scholar]
[33]. Schwebke JR, Desmond R, Natural history of asymptomatic bacterial vaginosis in a high-risk group of womenSex Transm Dis 2007 34(11):876-77. [Google Scholar]
[34]. Schwebke JR, Richey CM, Weiss HL, Correlation of behaviors with microbiological changes in vaginal floraJ Infect Dis 1999 180(5):1632-36. [Google Scholar]
[35]. Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK, Anaerobic bacteria in nonspecific vaginitisN Eng J Med 1980 303(11):601-07. [Google Scholar]
[36]. Hillier SL, The complexity of microbial diversity in bacterial vaginosisN Eng J Med 2005 353(18):1886-87. [Google Scholar]
[37]. Hill GB, The microbiology of bacterial vaginosisAm J Obstet Gynaecol 1993 169(2):450-54. [Google Scholar]
[38]. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK, Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associationsAm J Med 1983 74(1):14-22. [Google Scholar]
[39]. Barbone F, Austin H, Louv WC, Alexander WJ, A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis, and bacterial vaginosisAm J Obstet Gynaecol 1990 163(2):510-14. [Google Scholar]
[40]. Wølner-Hanssen P, Eschenbach DA, Paavonen J, Stevens CE, Kiviat NB, Critchlow C, Association between vaginal douching and acute pelvic inflammatory diseaseJAMA 1990 263(14):1936-41. [Google Scholar]
[41]. Leitich H, Kiss H, Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcomeBest Pract Res Clin Obstet Gynaecol 2007 21(3):375-90. [Google Scholar]
[42]. Martin DH, The microbiota of the vagina and its influence on women’s health and diseaseAm J Med Sci 2012 343(1):2 [Google Scholar]
[43]. Shipitsyna E, Roos A, Datcu R, Hallén A, Fredlund H, Jensen JS, Composition of the vaginal microbiota in women of reproductive age–sensitive and specific molecular diagnosis of bacterial vaginosis is possible?PloS one 2013 8(4):e60670 [Google Scholar]
[44]. Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Association between bacterial vaginosis and preterm delivery of a low-birth-weight infantN Eng J Med 1995 333(26):1737-42. [Google Scholar]
[45]. Pawanarkar J, Chopra K, Prevalence of lower reproductive tract infection in infertile womenHealth Popul Perspect Issues 2004 27(2):67-75. [Google Scholar]
[46]. Sharma S, Gupta B, The prevalence of reproductive tract infections and sexually transmitted diseases among married women in the reproductive age group in a rural areaIndian J Community Med 2009 34(1):62-64. [Google Scholar]
[47]. Bang RA, Baitule M, Sarmukaddam S, Bang AT, Choudhary Y, Tale O, High prevalence of gynaecological diseases in rural Indian womenLancet 1989 333(8629):85-88. [Google Scholar]
[48]. Luthra UK, Mehta S, Bhargava NC, Ramachandran P, Murthy MN, Sehgal A, Reproductive tract infections in India: The need for comprehensive reproductive health policy and programsIn Reproductive Tract Infections. Springer US 1992 :317-342. [Google Scholar]
[49]. Priestley CJ, Jones BM, Dhar J, Goodwin L, What is normal vaginal flora?Genitourin Med 1997 73(1):23-28. [Google Scholar]
[50]. Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, The preterm prediction study: significance of vaginal infectionsAm J Obstet Gynaecol 1995 173(4):1231-35. [Google Scholar]
[51]. Venugopal S, Gopalan K, Devi A, Kavitha A, Epidemiology and clinico-investigative study of organisms causing vaginal dischargeIndian J Sex Transm Dis 2017 38(1):69 [Google Scholar]
[52]. Thulkar J, Kriplani A, Agarwal N, Vishnubhatla S, Aetiology & risk factors of recurrent vaginitis & its association with various contraceptive methodsIndian J Med Res 2010 131(1):83-87. [Google Scholar]
[53]. Ratnaprabha GK, Thimmaiah S, Johnson AR, Ramesh N, Prevalence and awareness of reproductive tract infections among women in select underprivileged areas of Bangalore cityInt J Med Sci Pub Health 2015 4(12):1691-96. [Google Scholar]
[54]. Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD, Maternal stress is associated with bacterial vaginosis in human pregnancyMaternal And Child Health Journal 2001 5(2):127-34. [Google Scholar]
[55]. Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA, The association of psychosocial stress and bacterial vaginosis in a longitudinal cohortAm J Obstet Gynaecol 2006 194(2):381-86. [Google Scholar]
[56]. Khamees SS, Characterization of vaginal discharge among women complaining of genital tract infectionInt J Pharm & Life Sci 2012 3(10) [Google Scholar]