Introduction
Periodontitis, is an immune inflammatory response which arises from the interaction between the periodontopathogenic bacteria and host [1]. The course of periodontal disease is marked by discontinuous pattern of disease activity and inactivity showing exacerbation and remission [2,3]. The traditional clinical assessment methods include attachment level, probing depth, bleeding on probing, radiographic assessment of alveolar bone loss, but they neither provide information on the measures of disease activity nor do they identify the individuals who are susceptible to future disease progression [4,3] as the biologic phenotypes are not reflected properly in the clinical phenotype [1]. Biological phenotypes may then be taken into consideration which will be of help in assessing the burden of microbial and inflammatory load, which further affects the progression of periodontitis [5]. Earlier the disease is diagnosed, more likely it is to be cured successfully [6].
It is essential to know the biologic process for the development of new diagnostic tests which help in locating the sites with active disease and predicting future progression of disease. All this helps in evaluating the patient’s response to periodontal therapy [7]. Field of medicine commonly uses oral fluid–based POC diagnostics and lately it is being employed as the potential “chairside” test for determination of oral diseases [8]. POC testing can be defined as testing performed close to the patient at the time care is required [9]. Development of microfluidic approaches and detection of biomarker molecules in the oral cavity using advanced techniques like PCR for RNA and DNA and ELISAs for proteins, makes oral-based POC methods for the diagnosis a reality [10]. Thus, POC diagnostic testing is about to revolutionize the periodontal diagnostics and the therapy. This review is an overview on the periodontal diagnosis and focuses on the POC diagnostics and its application in periodontics. For better understanding of the topic, various flow-diagrams have been used to explain the principle behind the use of each test kit.
Need for A Periodontal Diagnostic Indicator
For any clinician, the greatest challenge is the identification of patients with high risk of active disease and also the active periodontal disease sites. There is great requirement for new research in the field of diagnosis that will help in the early recognition of the microbial challenge, presence of disease process, vulnerable sites for future breakdown and assess patient’s response [11].
The principles of these new diagnostic tests largely rely on the detection of the markers of disease activity. The term "markers of disease" basically consists of three separate categories; (1) indicators of current disease activity; (2) predictors of future disease progression; (3) predictors of initiation of future disease at a currently healthy site. It is also important to define "disease", so as to separate gingivitis from destructive periodontitis [12].
Biomarkers are quantifiable and measurable biologic parameters that serve as indicator for health and physiology-related assessments [13]. The biomarkers provide “signature” of the health state; they are found in the biological fluids such as blood, urine and more recently saliva [14]. It is rightly said that oral fluid is the mirror of periodontal health and it serves as medium to provide clinically relevant information since it contains biomarkers specific for periodontal diseases. Some of the oral fluid biomarkers like proteins of host origin (e.g., enzymes and immunoglobulins), host cells (e.g., PMNs), bacteria as well as bacterial products, ions, hormones and volatile compounds have been studied for periodontal diagnosis [15].
A number of diagnostic tests relying on physical, chemical, microbiological and immunological methodologies have emerged in the last decade [16]. Because of the unpredictable pattern of disease and presence of risk factors associated with the disease. these tests should not only provide valuable information on the initial diagnosis but also helps in the long term maintenance of periodontal patients [17]. This innovation in diagnosis would ultimately be a boon for the clinical management of periodontal patients.
Point of Care Diagnostics
High specificity and sensitivity are essential requirements of a good diagnostic marker which could be used chairside or in a home use device. The widespread use, simplicity, level of reliability and relative low cost of a home-used pregnancy test is the path to follow in periodontics [18].
New technologies like ‘lab-on-a-chip’ and microfluidic devices have emerged as a great hope in managing oral fluids such as saliva and gingival crevicular fluid and they also determine patient’s periodontal disease-risk profile, current disease activity and response to therapeutic interventions. This approach in turn proves to be useful in a chronic infectious disease such as periodontitis in terms of monitoring of episodic nature of this disease and in making clinical decision [11]. The various vehicles used for assessing periodontal disease activity are saliva, serum, GCF but because saliva and GCF are fluids that can be collected with ease and are rich in locally and systemically derived markers of periodontal disease, these hold a great potential for the assessment of patient-specific biomarker in the diagnosis of periodontitis and other systemic diseases[7].
Saliva
Saliva is a biofluid which is readily accessible and can be collected by totally non-invasive method. Variety of substances may enter saliva from the blood by passing through cells by passive diffusion and active transport, or by extracellular ultrafiltration inside the salivary glands or via the gingival sulcus [Table/Fig-1]. So, most compounds found in blood are also present in saliva. Saliva is a very useful and easily accessible body fluid which can be used to monitor oral and systemic health [13].
Table Demonstrating the biomarkers present in saliva.
| Markers of periodontal soft tissue inflammation | Markers of alveolar bone loss | Collagen breakdown products |
|---|
| Prostaglandin E2 | Alkaline phosphatase | Aspartate aminotransferase |
| β-glucuronidase | Osteoprotegerin | Alanine aminotransferase |
| IL-1β | Osteocalcin | TIMPs |
| IL-6 | Collagen telopeptidase | MMPs |
| Tumor necrosis factor-α | Pyridinoline cross-links of type I collagen | α2-macroglobulin |
| Matrix Metalloproteinase (MMP-8,9 and 13) | RANKL | |
| Osteonectin | |
Whole saliva of patient with oral diseases is rich in various mediators of chronic inflammation and tissue destruction [11]. More than 1,000 proteins have been detected in saliva as biomarkers [19]. With the detection of small quantities of salivary components like proteins and messenger RNA (mRNA), salivary diagnostics is currently one of the most promising areas of research in dentistry. [20,21]
Use of saliva in point of care diagnostics: Saliva offers many advantages as it is readily available, contains a rich array of diagnostic biomarker molecule, non-invasive method of sampling and ability to obtain rapid and reliable results [Table/Fig-2]. Saliva has also proved to be beneficial as compared to blood because it is easy to handle saliva as it does not clot and also chances of accidental transmission of infectious disease during its collection is less than blood samples. [19] However, one of the major limitations of using saliva is that as compared to saliva and serum the informative analytes generally are present in lower amount therefore, assays need to be highly sensitive [22]. The origin of saliva determines its composition and is influenced by various environmental and psychological stimuli. Thus, qualitative analysis of saliva markers can be reliably achieved but to quantify these markers is the real problem. Apart from these, presence of mucins and cell debris makes saliva a challenging fluid to work with.
Commercially available point of care diagnostics.
| Test Kits | Functions |
|---|
| Oral fluid nanosensor test | Detection of multiple salivary proteins and nucleic acids. |
| Electronic taste chips | Simultaneously monitor several biomarkers related to periodontal disease |
| OraQuick | Usually detects HIV 1 and HIV 2 |
| Integrated microfluidic platform for oral diagnostics | Quantification of an oral disease biomarker |
Biochemical Test [Table/Fig-2]
a. Oral fluid nanosensor test: A new POC device to detect oral cancer in saliva was developed by the University of California, Los Angeles (UCLA) Collaborative Oral Fluid Diagnostic Research Laboratory, led by Dr. David Wong [23, 13]. This is an automated POC device that is designed for the electrochemical detection of multiple salivary proteins and nucleic acids. It is an ultra-sensitive and ultraspecific micro electromechanical system which simultaneously and precisely detects these proteins and nucleic acid. The product is Oral Fluid Nano Sensor Test (OFNASET). Four salivary mRNA biomarkers (SAT, ODZ, IL-8 and IL-1b) and two salivary proteomic biomarkers (thioredoxin and IL-8) in saliva are detected in this system [19]. The OFNASET is actually a screening device for detecting oral cancer [23]
b. Electronic taste chips: Researchers at Rice University in Houston, Texas, are developing a lab-on-a-chip system, which will differentiate between healthy and periodontally diseased individuals based on the CRP levels [19]. This microchip based detection system is used for measuring analytes (acids, bases, electrolytes and proteins) in solution phase. This novel system is called an Electronic Taste Chip (ETC). On the interior regions of the microspheres, sensor array platform is placed where all the chemical and immunological reactions are performed. These microspheres are located on the inverted pyramidal microchambers of microchip. A Charge-Coupled Device (CCD) video chip visualizes and captures the various optical signals generated by the reactions on the microspheres. The ETC system has the advantage over the ELISA in having porous beads, which allows greater number of antibody molecules to capture and thus detect, CRP at extremely low concentrations. In ELISA, antigen–antibody interactions are generated on a single layer at the bottom of the well [24].
c. OraQuick: To expedite screening and accurately diagnose HIV infection, rapid POC HIV tests have been developed [25]. which provides results in 20 minutes. The fluid to be diagnosed is mixed in a vial with developing solution and the results are displayed on a testing device. It is a stick-like device with a fabric swab on one end which is inserted into a tube of testing fluid [19]. OraQuick® is the first FDA-approved oral swab in-home test for HIV-1 and HIV-2.
d. Integrated microfluidic platform for oral diagnostics (IMPOD): IMPOD, a POC diagnostic test, helps in the rapid quantification of salivary biomarkers related to oral disease. It facilitates hands-free saliva analysis by integrating sample pretreatment with electrophoretic immunoassays to quickly measure analyte concentrations in minimally pretreated saliva samples. Rapid measurement of levels of the collagen cleaving enzyme MMP-8 in saliva from healthy and periodontally diseased subjects can be achieved. The hand-held IMPOD has been used to rapidly (3–10 minutes) measure the concentrations of MMP-8 and other biomarkers in small amounts (10 ml) of saliva [19].
Microbiological Test
a. My PerioPath: My PerioPath detects the pathogens causing periodontal disease in saliva samples. This test uses DNA polymerase chain reaction to detect the type and concentration of bacteria present in the salivary sample [26].
b. Omnigene: OmniGene Diagnostics, Inc. are species specific DNA probes to identify eight pathogens which are known to cause periodontal disease, (Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetem-comitans, Fusobacterium nucleatum, Eikenella corrodens, Campylobacter rectus, Bacteroides forsythus and Treponema denticola). The advantage of using these test kits is that the results are available in short period of time and can be mailed or faxed to the clinician [27].
This is a microbiological test which detects microrganisms causing periodontitis like A. actinomycetemcomitans, P. gingivalis, T. forsythia and T. denticola using RNA probes in the sample collected. [26]
Genetic Test
a. MyperioID: MyPerioID identifies the genetic susceptibility of the patient to periodontal diseases by using salivary samples which are shipped to the laboratory for the results. These test plays role in evaluating the patients which are at higher risk of periodontal destruction [26].
Gingival Crevicular Fluid (GCF)
GCF, is a body fluid derived from serum, leukocytes and cells of the periodontium and oral microflora [28]. Its composition is the result of the interplay between the bacterial biofilm and the cells of the periodontal tissues [29]. The specific composition of GCF is the biochemical indicator of the locally produced changes in metabolism, thus determining the periodontal status of the individual. Since host response is a critical determinant in periodontal disease pathogenesis, inflammatory mediator levels in the GCF can be used to evaluate ‘risk’: risk for a tooth, or more precisely a site where clinical attachment and alveolar bone may be lost, or risk for an individual to develop periodontal disease [29]. GCF contains a variety of potential markers derived from host and bacteria from supragingival and subgingival plaque thus, offering a wide array of candidate makers for detection of periodontal disease activity [Table/Fig-3].
Biomarkers in gingival crevicular fluid.
| Inflammatory and immune products | Bacterial proteases | Host derived enzymes | Tissue breakdown products | Bone specific proteins |
|---|
| Prostaglandin E2 (PGE2) | Alkaline phosphatase | Alkaline phosphatase | Glycosamin oglycan | Pyridinium crosslink urine pyridinoline |
| Cytokines | Aminopepti dases | β-Glucuroni dase | Hyaluronic acid | Pyridinium cross-link collagen peptide fragment |
| Antibacterial antibodies | Chondroitin sulphatase | Elastase | Chondroitin-4-sulfate | Tartrate-resistant acid phosphatase |
| Acute phase proteins | Collagenase | Cathepsins | Chondroitin-6-sulfate | Hydroxyproline |
| Complement | Fibrinolysin | Serine prote nase (G) | Dermatan sulfate | Galactosyl hydroxylysine |
| Vasoactive intestinal | Glucosidases | Nonspecific neutral | Hydroxyp roline | Glycosaminog lycans |
| Peptide | | proteinases | | |
| Neurokinin a | Hemolysin | Matrix metalloproteinase-l,3,8,13 | Fibronectin fragments | Osteonectin and bone phosphoprotein |
| Neopterin | Hyaluronidase | Aspartate amino transferase | Connective tissue and bone proteins | osteocalcin |
| Platelet -activating factor | Phospholipase | Myeloperoxidases | Type I collagen peptides | |
| Hydroxyproline | Lactate dehydrogenase | Polypeptide growth factor | |
The gingival fluid is potential medium for the detection of early changes which could indicate the onset of disease. [18] According to Zia A et al., more than 65 chemicals of GCF have been considered as future markers of prognosis of various disease of periodontium [30].
These biomarkers can be further divided into 5 groups: i) Inflammatory and immune products ii) Bacterial enzymes iii) Host derived enzymes iv) Tissue breakdown products and v) Bone specific proteins.
Use of GCF in point of care diagnostics: GCF can be frequently used for biomarkers as it easily obtained from the oral cavity. Chapple I stated the advantages of using GCF: "The biomarkers found in GCF indicate the presence or absence of periodontal pathogens, gingival and periodontal inflammation, the host inflammatory-immune response to specific pathogenic species and host tissue destruction". The disadvantages of using GCF are that it requires multiple samples of individual tooth sites and extensive laboratory processing, thereby making it expensive and time consuming [31].
Although, GCF has several diagnostic advantages because of the appearance of inflammatory mediators and tissue-destructive molecules in it, the procedure of collection and analysis makes it difficult to be used as a chairside diagnostic medium [Table/Fig-4]. GCF collection is laborious and technically demanding requiring special equipment for calibrating and measuring fluid volumes. There is also a possibility of GCF being contaminated with blood, saliva, or plaque [25].
Commercially available kits using GCF for detecting host derived enzymes.
| Test Kits | Enzymes |
|---|
| Periogard | AST |
| Pocket watch | AST |
| Periocheck | Collagenase (neutral protease) |
| Prognostik (Dentsply), Biolise | Elastase (serine protease) |
| MMP dipstick method | MMP |
Biochemical Test
a. Periogard: Aspartate aminotransferase (AST) which is released on cell death is the main enzyme that is detected by PerioGard [Table/Fig-5]. In periodontal diseases due to cell death there is elevated AST levels which act as a positive marker in active locations [32,33]. The test contains two wells for each for tooth and the chemicals [34].
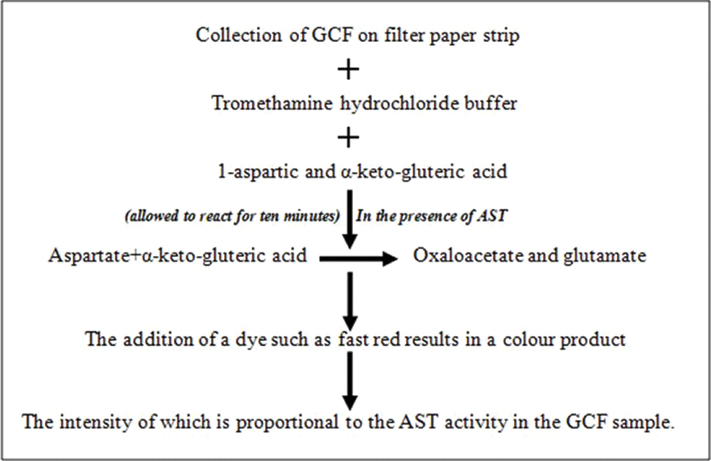
But in practice, PerioGard assay is a relatively complex process which involves numerous steps and has difficulty in color measurement.
b. Pocket watch: The Pocket watch is a chairside test for analyzing AST levels [35].
Principle: AST acts as catalyst in the exchange of an amino group of cysteine sulfuric acid by α- keto- gluteric acid to produce β-sulfinyl pyruvate in the presence of pyridoxal phosphate. Inorganic sulphite is released by the spontaneous decomposition of glutamate β-sulfinyl pyruvate. The sulfite ion thus produced reacts with Malachite Green (MG), which converts a green dye to its colorless form, thereby showing the pink–colored rhodamine B dye.
The AST concentration can be assessed though the rate of conversion of MG.
c. Periocheck: Periocheck is a Food and Drug Administration (FDA) approved product [Table/Fig-6] [36].
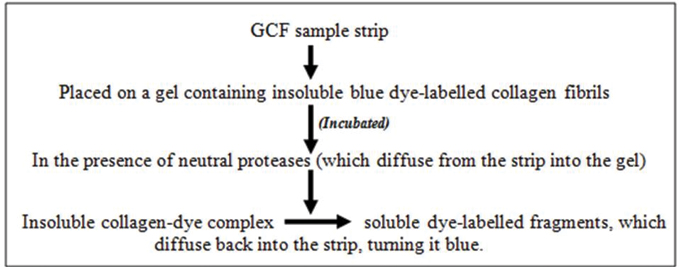
Periocheck is the most rapid chairside test for detecting neutral proteases in GCF like elastases, proteinases and collagenase, still it suffers from certain drawbacks like interproximal sites cannot be sampled due to saliva contamination, test is not specific for PMNL collagenase and may include enzymes of bacterial origin.
d. Prognostik: Prognostik, developed in the year 1993, measures the levels of MMPs such as the elastases in the GCF [Table/Fig-7].
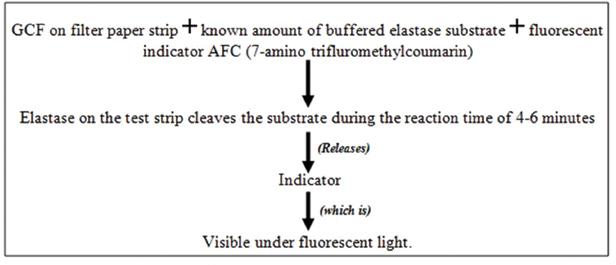
Active disease sites produce an elevated elastases level in the GCF as released from the lysosomes of polymorphonuclear leucocytes [37]. However, further clinical trials are required to establish relationship between elastase levels in GCF and periodontal disease activity.
e. MMP dipstick test: MMPs are host-derived proteinases which plays a major role in periodontitis and dental peri-implant health and diseases. This forms the basis for the development of both qualitative and quantitative chairside POC technologies which will help in the rapid detection of pathologically elevated levels of MMP-8 in oral fluids and serum. Monoclonal antibodies for MMP-8 are being utilized in chairside POC immunotests for oral fluid and serum MMP-8 analysis. The MMP-8 stick-test can differentiate healthy gingiva and gingivitis sites from periodontitis sites and the results obtained correlates with that of quantitative laboratory Immunofluorometric Assay (IFMA). [38]
Microbial test KITS
A plethora of research activity had explored the role of plaque as a possible medium for detecting the periopathogens which is an important aspect in the diagnosis and treatment of periodontal diseases [Table/Fig-8] [7]. Considerable newer developments have occurred in methods of detecting periodontopathogens in plaque samples [Table/Fig-9].
Biomarkers present in dental biofilm.
| Markers present in dental biofilm |
|---|
| Specific | Non-specific | Systemic |
| Immunoglobulins(IgA, IgG and IgM) | Mucins | C-Reactive Protein |
| Lysozyme |
| Lactoferrin |
| Histatin |
| Peroxidase |
Other commercially available kits for detecting bacterial protease.
| Test kits | Bacteria and their products |
|---|
| Perioscan (BANA test)Oral B lab | Trypsin like protease |
| Evalusite (Kodak) | P. gingivalis, P. intermedia, A. actinomycetemcomitans |
| Perioscan/ Diamond probe/Probe 2000 system | For volatile sulphur compounds |
| TOPAS | Bacterial toxins and protease |
a. Perioscan (BANA):P. gingivalis, T. denticola, T. forsythia and some Capnocytophaga strains produce bacterial trypsin-like proteases in the dental plaque which can be detected by Perioscan [Table/Fig-10] [39,40].
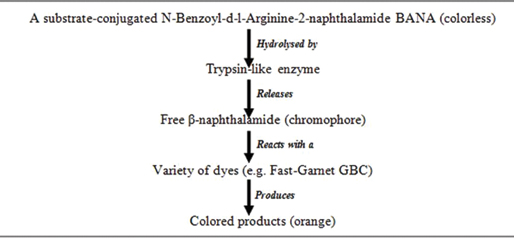
The major drawbacks of this test being that it cannot identify the pathogens which produces non-trypsin like enzymes and its inability to differentiate the specific bacteria amongst the three producing these enzymes.
b. Evalusite: Three putative periodontopathogens (Aa, Pg and Pi) can be detected using membrane-based enzyme immunoassay, Evalusite [Table/Fig-11].
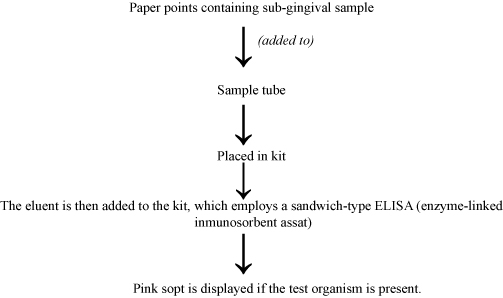
Subjective assessment of the color is one of the major disadvantages of this test. Also, this assumption that the three bacteria detected are the only disease causing organisms limits its use [41].
c. Perio 2000: Degradation of serum proteins (cysteine and methionine) leads to Volatile Sulphide Compounds (VSCs) production by microorganisms like P. gingivalis, P. intermedia and T. forsythia. Evaluations of VSCs are indicative of subgingival microbial load as it plays role in degrading periodontal structures aggravating periodontitis. Perio 2000 system displays the sulphide level digitally at each site. Sterile wash solution is used to hydrate the tip then at peak or hold operational mode it is inserted subgingivally. After obtaining the reading, the tip is washed and reinserted in other subgingival site.
d. Toxicity Prescreening Assay (TOPAS): TOPAS is a chairside test kit for indirectly detecting bacterial toxins and bacterial proteins which are one of the markers for the presence of gingival infection. The principle behind this test relies on the detection of actively dividing and growing pathogens which can be assessed through the metabolic activity of these organisms in the crevicular fluid. This test can be used to know difference between an active and an inactive periodontal disease as indicated by the change in the color intensity scale of the test based on the fact that metabolic activity increases as the concentrations of these toxins increases [42].
Genetic Test
The Periodontitis Susceptibility Trait test (PST) is the test which identifies the genetic predisposition of the patient for periodontitis by detecting the polymorphism in IL-1 gene. Polymorphism in two positions of IL-1 i.e position -889 and + 3953 has been associated with periodontal disease [27].
Advantages of Point of Care
POC testing eliminates the need to draw blood and reduces the cost and inventory associated with sample shipping and handling to a centralized laboratory [19] thereby, reducing the total time involved and improves the quality of care delivered by allowing treatment to begin immediately. Periodontal oral POC diagnostic devices requires less training and fewer resources than current diagnostic tests, proving to be more effective, thereby enabling screening of large populations quickly. The benefit of screening various population is the identifying at-risk groups more effectively and increasing the access to treatment [11].
Disadvantages of Point of Care Diagnostics
The use of POC diagnostics in the periodontal surveillance looks promising; however in the clinical setting, these approaches suffer from various obstacles. These new periodontal diagnostics needs to be validated and benchmarked with existing methods of disease evaluation (alveolar bone levels and clinical attachment levels). Acceptance of such methods by dentists and treatment clinicians is imperative and may prove to be difficult.
Another issue to be addressed is the cost effectiveness of the procedure. Clinician needs to be abreast with the knowledge of diagnosis, disease risk and its prevention before diagnostics may be integrated into routine clinical periodontal practice [11].
Al, the above describes oral tests has been summarised in [Table/Fig-12].
Summarisation of oral tests.
| S. No. | Oral fluid | Test | Kit |
|---|
| 1 | Saliva | Biochemical test | a) Oral fluid nanosensor testb) Electronic taste chipc) OraQuickd) Integrated microfluidic platform for oral Diagnostics |
| Microbiological test | a) My PerioPathb) Omnigenec) IAI pado test |
| Genetic test | a) MyperioID |
| 2. | Gingival crevicular fluid | Biochemical test | a) Perriogardb) Pocket watchc) Periocheckd) Prognostice) MMP dipstick test |
| 3. | Plaque | Microbiological test | a) Perioscan (BANA)b) Evalusitec) Perio 2000d) TOPASGenetic test kits |
| 4. | Periodontitis susceptibility trait test |
Advances in Point of Care Diagnostics-Lab-On-Chip Devices
A newer generation of POC technology called lab-on-a-chip is under the process of development [19]. This is basically a device which integrates and automates all the complexities of a laboratory procedure into a chip of a size of computer chip [36]. This technology seeks to measure multiple biomarkers in a small saliva sample [23,36].
Conclusion
An accurate initial diagnosis is a cornerstone for the success of any periodontal treatment, existing diagnostic methods suffices the purpose but it is desirable to assess the “active disease sites”. With the advent of new commercially available chairside test kits [Table/Fig-7] which uses host and bacterial markers of periodontal disease monitoring of specific sites is now possible. Great amount of research activity is being undertaken to investigate the role of oral fluids as a medium for diagnostic purposes in various fields. Although, challenges remain ahead, the use of saliva and GCF based oral fluid diagnostics are promising in the diagnosis of periodontal diseases and predicting periodontal treatment outcomes. Although, challenges remain ahead, the use of saliva and GCF based oral fluid diagnostics are promising in the diagnosis of periodontal diseases and predicting periodontal treatment outcomes.
[1]. Hernández M, Vernal R, Sorsa T, Tervahartiala T, Mäntylä P, Gamonal J, The Role of immuno-inflammatory response in the pathogenesis of chronic periodontitis and development of chair-side point of care diagnostics. In Prof. Nurcan Buduneli, editorPathogenesis and Treatment of Periodontitis [Internet] 2012 InTech Publishing Co.Chapter 3 [Google Scholar]
[2]. Offenbacher S, Periodontal diseases: PathogenesisAnn Periodontol 1996 1:821-78. [Google Scholar]
[3]. Sahingur SE, Cohen RE, Analysis of host responses and risk for disease progressionPeriodontol. 2000 2004 34:57-83. [Google Scholar]
[4]. Position paper: Diagnosis of periodontal diseaseJ Periodontol 2003 74:1237-47. [Google Scholar]
[5]. William V, Giannobile Salivary diagnostics for periodontal diseasesJADA 2012 143:6S-11S. [Google Scholar]
[6]. Lee Y, Wong DT, Saliva: An emerging biofluid for early detection of diseaseAm J Dent 2009 22:241-48. [Google Scholar]
[7]. Taba M Jr, Kinney J, Kim AS, Giannobile WV, Diagnostic biomarkers for oral and periodontal diseasesDent Clin North Am 2005 July 49:551-72. [Google Scholar]
[8]. Tabak LA, Point-of-care diagnostics enter the mouthAnn N Y Acad Sci 2007 1098:7-14. [Google Scholar]
[9]. Price Christopher P, John Andrew St, Hicks Jocelyn M, Point of Care Testing 2004 2nd editionWashington, DCAACC Press:506 [Google Scholar]
[10]. Giannobile WV, McDevitt JT, Niedbala RS, Malamud D, Translational and clinical applications of salivary diagnosticsAdv Dent Res 2011 23:375-80. [Google Scholar]
[11]. Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT, Saliva as a diagnostic tool for periodontal disease: current state and future directionsPeriodontology 2000 2009 50:52-64. [Google Scholar]
[12]. Curtis MA, Gillett IR, Griffiths GS, Maiden MF, Sterne JA, Wilson DT, Detection of high-risk groups and individuals for periodontal diseases: Laboratory markers from analysis of gingival crevicuiar fluidJ Clin Periodontol 1989 16:01-11. [Google Scholar]
[13]. Spielmann N, Wong DT, Saliva: diagnostics and therapeutic perspectivesOral Diseases 2011 17:345-54. [Google Scholar]
[14]. Wei F, Wong DT, Point-of-care platforms for salivary diagnosticsChinese J Dent Res 2012 15:07-15. [Google Scholar]
[15]. Cafiero C, Matarasso S, Predictive, preventive, personalised and participatory periodontology: ‘The 5Ps age’ has already startedThe EPMA Journal 2013 4(16):01-29. [Google Scholar]
[16]. Kinane DF, Regulators of tissue destruction and homeostasis as diagnostic aids in periodontologyPeriodontology 2000 2009 24:215-25. [Google Scholar]
[17]. Chapple I, Periodontal disease diagnosis: Current status and future developmentsJ Dent 1997 :2503-15. [Google Scholar]
[18]. Loos BG, Tjoa S, Host-derived diagnostic markers for periodontitis: do they exist in gingival crevice fluid? Periodontol 2000 2005 39:53-72. [Google Scholar]
[19]. Priyanka N, Kalra N, Shanbhag N, Kumar K, Seema Brijet B, Recent approaches in saliva as a credible periodontal diagnostic and prognostic markerAOSR 2012 2:40-46. [Google Scholar]
[20]. Kirk EC, Saliva as an index of faulty metabolismDent Diag 1903 9:1126-38. [Google Scholar]
[21]. Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, The proteomes of human parotid and submandibular/ sublingual gland salivas collected as the ductal secretionsJ Proteome Res 2008 7:1994-06. [Google Scholar]
[22]. Miller SM, Saliva testing: A non-traditional diagnostic toolClin Lab Sci 1994 7:3944 [Google Scholar]
[23]. Wong DT, SalivaomicsJ. Am Dent Assoc 2012 143:19S-24S. [Google Scholar]
[24]. Christodoulides N, Tran M, Floriano PN, Rodriguez M, Goodey A, Ali M, A microchip-based multianalyte assay system for the assessment of cardiac riskAnal Chem 2002 74:3030-36. [Google Scholar]
[25]. Patil PB, Patil BR, Saliva: A diagnostic biomarker of periodontal diseasesJ Ind Soc Periodontol 2011 15:310-17. [Google Scholar]
[26]. Chepuri T, Gooty JR, Durvasala S, Palaparthi R, Chair side diagnostic test kits in periodonticsIndian J Dent Adv 2015 7:41-45. [Google Scholar]
[27]. Pajnigara NG, Kolte AP, Kolte RA, Pajnigara NG, Chair side diagnostic kits in periodonticsInternational Dental Journal of Student’s Research 2016 4:25-31. [Google Scholar]
[28]. Uitto VJ, Gingival crevice fluid – an introductionPeriodontology 2000 2003 31:09-11. [Google Scholar]
[29]. Champagne CME, Buchanan W, Potential for gingival crevice fluid measures as predictors of risk for periodontal diseasesPeriodontol 2000 2003 31:167-80. [Google Scholar]
[30]. Zia A, Khan S, Bey A, Gupta ND, Mukhtar-Un-Nisar S, Oral biomarkers in the diagnosis and progression of periodontal diseasesBiology and Medicine 2011 3:45-52. [Google Scholar]
[31]. Chapple I, Periodontal diagnosis and treatment — where does the future lie?Periodontol 2000 2009 51:09-24. [Google Scholar]
[32]. Persson GR, DeRouen TA, Page RC, Relationship between levels of aspartate aminotransferase in gingival crevicular fluid and active tissue destruction in treated chronic periodontitis patientsJ Periodont Res 1990 25:81-87. [Google Scholar]
[33]. Chambers D, Imrey P, Cohen R, Crawford J, Alves M, Mcswiggin T, A longitudinal study of aspartate aminotransferase in human gingival crevicular fluidJ Periodont Res 1991 26:65-74. [Google Scholar]
[34]. Persson GR, Alves ME, Chambers DA, Clark WB, Cohen R, Crawford JM, A multicentered clinical trial of PerioGard in distinguishing between diseased and healthy periodontal sitesJ Clin Periodontal 1995 22:794-803. [Google Scholar]
[35]. Shimhadaka K, Mizuno T, Analysis of AST in gingival crevicular fluid assessed using Perio Watch: A longitudinal study with initial therapyJ Clin Periodontal 2000 27:819-23. [Google Scholar]
[36]. Chapple I, Matthews J, Thorpe G, Glenwright H, Smith J, Saxby M, A new ultrasensitive chemiluminescent assay for the site-specific quantification of alkaline phosphatase in gingival crevicular fluidJ Periodont Res 1993 28:266-73. [Google Scholar]
[37]. Armitage GC, Jeffcoat MK, Chadwick DE, Taggart EJ Jr, Numabe Y, Landis JR, Longitudinal evaluation of elastase as a marker for the progression of periodontitisJ Periodontol 1994 65:120 [Google Scholar]
[38]. Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclinesPharmacological Research 2011 63:108-13. [Google Scholar]
[39]. Laughton B, Syned S, Loesche W, API ZYM system for identification of Bacteroides ssp., Capnocytophagia ssp., and spirochaetes of oral originJ Clin Microbial 1982 15:97-127. [Google Scholar]
[40]. Loesche W, Syed S, Stoll J, Trypsin-like activity in sub-gingival plaque: A diagnostic marker for spirochetes and periodontal disease?J Periodont 1987 58:266-73. [Google Scholar]
[41]. Mikx FH, Renggli HH, How sensible are bacteriological tests in periodontology? Ned Tijdschr Tandheelkd 1994 101:484-88. [Google Scholar]
[42]. Puscasu CG, Dumitriu A, Dumitriu HT, Biochemical and enzymatic diagnosis aids in periodontal diseaseOHDMBSC 2005 4:19-25. [Google Scholar]