Pleomorphic Hyalinizing Angiectatic Tumour (PHAT) is one of the rare soft tissue tumour which is non-metastasizing. The origin of this tumour is yet uncertain. It occurs in adults as a slow growing subcutaneous mass mimicking clinically and histologically to various benign and malignant soft tissue tumours such as schwannoma, haemangioma and malignant fibrous histiocytoma. The microscopic features of this tumour include clusters of ectatic, fibrin containing, hyalinized blood vessels with pleomorphic and spindle shaped tumour cells showing intranuclear inclusions, stromal haemosiderin pigment and a variable inflammatory infiltrate. Despite marked pleomorphism, the lesion behaves as a low grade neoplasm, with frequent recurrences, but no metastases. The incidence of this tumour is very rare with less than 100 cases being published. Hence, awareness of this entity is must for proper management of the patient and to avoid misdiagnosis of the lesion. We report a case of pleomorphic hyalinizing angiectatic tumour in a 50-year-old man who presented with a slow growing mass in the left calf region since two years.
Ectactic vessels, Haemangioma, Malignant fibrous histiocytoma, Non- metastasizing, Schwannoma
Case Report
A 50-year-old man presented with a solitary, slow growing mass in the left calf region since two years. The patient gave history of rapid increase in size of the swelling since two months following thorn injury. Magnetic Resonance Imaging (MRI) revealed a large well defined lobulating heterogenous soft tissue malignant neoplasm arising from posteromedial aspect of gastrocnemius muscle extending into subcutaneous plane. Fine Needle Aspiration Cytology (FNAC) of the swelling revealed a low cellular smear with bland spindle shaped cells having moderate amount of cytoplasm, elongated nuclei and inconspicuous nucleoli admixed with anastomosing capillary network and few mature adipocytes in a haemorrhagic background. Based on these findings differential diagnosis of haemangioma or angiolipoma were given.
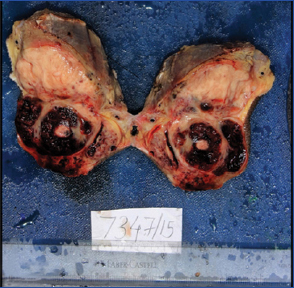
Wide surgical excision of the mass was done and sent for histopathological evaluation. Grossly, the mass measured 13.5 x 12 x 6 cm with an ulcer on the skin surface measuring 6 x 5 cm. On cut surface, circumscribed, firm mass was noted measuring 9 x 5.5 cm, which was predominantly solid having lobulated appearance. Also, seen many blood filled cystic spaces of varying sizes [Table/Fig-1,2].
Gross appearance of the specimen with an ulcer on the skin surface;

Cut surface of the specimen shows variegated appearance with solid and blood filled cystic areas.
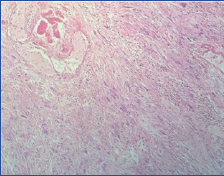
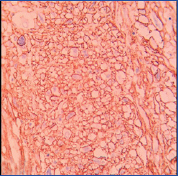
Microscopically, the lesion was unencapsulated with vascular and cellular areas. Cellularity was variable and it was composed of hyper and hypocellular areas. The tumour tissue in the hypercellular areas predominantly comprised of spindle shaped cells arranged in interlacing bundles and fascicles, with round to oval vesicular nuclei, inconspicuous nucleoli and moderate amount of eosinophilic cytoplasm. Few pleomorphic cells having vesicular single or multilobated nucleus with prominent nucleoli and abundant eosinophilic cytoplasm were also noted. Few cells showed intranuclear inclusions. Many multinucleated giant cells were also noted. Hypocellular areas were comprised of spindle shaped cells with round to oval nuclei having fine nuclear chromatin and moderate amount of eosinophilic cytoplasm and were dispersed in a myxoid background. These tumour cells were infiltrating the adjacent adipose tissue focally. The vascular areas were comprised of many, large clusters of ectatic thin walled blood vessels. Subendothelial fibrin deposition, organized luminal thrombus and perivascular hyalinization were noted in majority of the vessels. Intervening stroma was showing dense and diffuse mixed inflammatory cell infiltrate composed of lymphocytes, plasma cells, few haemosiderin laden macrophages and mast cells. Mitotic figures were occasional. Based on the above features, diagnosis of PHAT was given [Table/Fig-3,4].
Microphotograph showing tumour tissue arranged in interlacing fascicles and bundles intermixed with variable sized capillaries showing organized luminal thrombus in few of them (H&E 20X);
Microphotograph showing pleomorphic cells and few multinucleated cells in a background of inflammatory cells (H&E 40X);
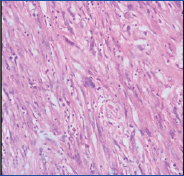
Further on Immunohistochemistry (IHC), the tumour cells showed strong membranous positivity for CD34. CD34 also highlighted the characteristic vasculature of the tumour [Table/Fig-5]. Tumour tissue was negative for S-100.
CD34 showing strong membrane positivity and characteristic vasculature (20X).
Even though PHAT presents with frequent recurrences, follow-up of the case for one year did not show any new symptoms or swelling.
Discussion
PHAT of soft parts is a rare tumour of intermediate malignancy first described by Smith ME et al., in 1996 [1]. In WHO classification of soft tissue tumours, this tumour is categorized under benign tumour of uncertain differentiation [2]. PHAT is a rare soft tissue neoplasm occurring within the superficial subcutaneous tissues and muscles [3,4]. Since its recognition, many published articles were either single case reports or small case series. To the best of our knowledge, study done by Folpe AL and Weiss SW is the largest of so far published articles with a total of 41 cases in a period of 10 years [5]. The most common site of predilection is lower extremity. Other less frequently affected sites include trunk, upper extremities, axilla, inguinal region, perineum, buttock, back, oral cavity, male breast and mesorectal soft tissue [3,4,6]. In the present case, lower extremity was affected which is the most common site for PHAT. It affects individuals with a broad age group between 10 to 89 years of age with a median age of 56 years [6,7]. Females are more commonly affected than males with female to male ratio of 4:3 [4]. However, in the present case the patient was 50-year-old male. PHAT presents as a slow growing, painless, non-tender, unencapsulated mass with size varying from less than 1cm to almost up to 20cm. Grossly, the tumours are unencapsulated, well circumscribed, firm mass having a lobulated appearance and a variegated cut surface with tan to maroon in colour [4,8]. The characteristic histological features of PHAT include clusters of varying sized thin walled ectatic blood vessels which show intra luminal and subendothelial fibrin deposition, luminal thrombi and perivascular hyalinization. These blood vessels are scattered in a stroma composed of sheets and fascicles of spindle shaped cells, pleomorphic cells and multinucleated giant cells containing intranuclear inclusions. The cells close to the vessels also contain fine haemosiderin pigment deposition within their cytoplasm. Intervening stroma shows inflammatory cell infiltrate composed primarily of mast cells, occasional myxoid areas and focal calcification [4,5,7-9]. Despite high pleomorphism, the mitotic activity is minimal or absent [4,5,7-9]. All these features except calcification were noted in our case. In the present case, there was predominance of the vascular component as compared to the cellular component. Many ectatic vessels of varying sizes with areas of haemorrhage and organized thrombus in some vessels was the predominant histological feature in the present case. Hence on FNAC, aspirate was haemorrhagic which was masquerading to a vascular lesion and was misdiagnosed as vascular tumour suggestive of haemangioma.
Ultrastructurally, neoplastic cells in PHAT have features suggesting fibroblastic differentiation. They contain large numbers of cytoplasmic filaments which represent vimentin filaments [10].
The main differential diagnoses include Malignant Fibrous Histiocytoma (MFH) and neurilemmoma (schwannoma) [3]. The features of MFH include pleomorpic cells arranged in storiform pattern, high mitotic rate, no intranuclear inclusions and absence of CD34 expression. However, in the present case low mitotic activity and CD34 positivity was noted and MFH was ruled out. The characteristics of neurilemmoma include encapsulation of tumour with palisading of nuclei, verocay body formation and S100 expresion of the tumour cells which were absent in the present case, hence neurilemmoma was excluded [3]. Other differential diagnoses include solitary fibrous tumour, giant cell angiofibroma, kaposi sarcoma and malignant melanoma [4].
Solitary fibrous tumour is characterized by alternating hypo and hypercellular areas, round to spindle cells with indistinct borders, vesicular nuclei and scant cytoplasm separated by striking areas of hyalinization, dense collagen and haemangiopericytoma like vessels [4,10]. Kaposi sarcoma features proliferation of small vessels surrounding ectatic vessels, bland looking spindle cells separated by slit like spaces containing erythrocytes, haemosiderin pigment, characteristic hyaline globules and expression of CD31 [4,10]. Giant cell angiofibroma is usually seen in orbital region and displays pseudovascular spaces which result from loss of cellular and stromal cohesion, bland round to spindle cells, collagenous or myxoid stroma and multinucleated floret like multinucleated stromal giant cells [4,10]. In our case haemangiopericytoma like vessels, hyaline globules and floret like giant cells were absent.
Smith ME et al., postulated that the characteristic ectatic vessels were secondary to engulfment of the vessels by the advancing tumour cells resulting in vascular damage, plasma leakage and perivascular hyalinization [1]. Another possibility for vascular leakage could be the release of vaso-active substances by the mast cell during tissue injury by tumour infiltration.
Folpe AL and Weiss SW proposed that the vascular changes occur very early even without a significant spindle cell proliferation [5]. Further, the characteristic pleomorphism of classic PHAT is a late, time dependent, degenerative phenomenon.
Groisman et al., hypothesized that the hypoxia induced by the hyalinized vessels increases VEGF production and further neovascularization [11].
In the present case the growth was since two years, hence as postulated by Smith ME et al., [1] and Folpe AL [5] and Weiss SW [10] the degenerative phenomenon may be the reason for pleomorphism and vascular changes noted in our case.
Conclusion
PHAT is a soft tissue tumour of low malignant potential histologically ranging from benign soft tissue tumour such as neurilemmoma to malignant soft tissue tumour such as malignant fibrous histiocytoma. Hence, awareness of this entity is must for proper diagnosis, management and follow-up. In our case follow up of the patient for one year of post surgery did not show recurrence of the tumour.
[1]. Smith ME, Fisher C, Weiss SW, Pleomorphic hyalinizing angiectatic tumour of soft parts: a low-grade neoplasm resembling neurilemomaAm J Surg Pathol 1996 20:21-29. [Google Scholar]
[2]. Fletcher CDM, Unni KK, Mertens F, World Health Organization Classifiication of TumorsPathology and Genetics of Tumors of Soft Tissue and Bone 2002 LyonIARC Press:191 [Google Scholar]
[3]. Suzuki K, Yasuda T, Hori T, Oya T, Watanabe K, Kanamori M, Pleomorhic hyalinizing angiectatic tumour arising in thigh: A case reportOncology Letters 2014 7(4):1249-52. [Google Scholar]
[4]. Raghavan V, Shivaprashanth K, Ramesh Rao K, Pleomorphic hyalinising angiectatic tumour: Immunohistochemical study with review of literatureIJSS Case Reports & Reviews 2015 1(8):46-49. [Google Scholar]
[5]. Folpe AL, Weiss SW, Pleomorphic hyalinizing angiectatic tumour: analysis of 41 cases supporting evolution from a distinctive precursor lesionAm J Surg Pathol 2004 28:1417?25 [Google Scholar]
[6]. Rekhi B, Aggarwal S, Pleomorphic hyalinizing angiectatic tumour exhibiting Intricate branching vasculature: An interesting pattern in a rare tumourIndian J Pathol Microbiol 2013 56:321-23. [Google Scholar]
[7]. Peng HC, Huang MT, Chen DJ, Leung TK, Chu JS, Pleomorphic hyalinizing angiectatic tumour of soft partsJ Formos Med Assoc 2010 109:616-20. [Google Scholar]
[8]. Lee JC, Jiang XY, Karpinski RHS, Moore ED, Pleomorphic hyalinizing angiectatic tumour of soft partsSurgery 2005 137:119-21. [Google Scholar]
[9]. Jaggon JR, Aitken RDC, Pleomorphic hyalinizing angiectatic tumour of soft parts- A case report and review of the literartureWest Indian Med J 2007 56:544-47. [Google Scholar]
[10]. Weiss SW, Goldblum JR, Folpe AL, Enzinger and Weiss’s soft tissue tumours 2014 6th EdPhiladelphiaElsevier:986-91. [Google Scholar]
[11]. Groisman GM, Bejar J, Amar M, Ben-Izhak O, Pleomorphic hyalinising angiectatic tumor of soft parts: immunohistochemical study including the expression of vascular endothelial growth factorArch Pathol Lab Med 2000 124:423-6. [Google Scholar]