A Rare Case of Pulmonary Lymphangitic Carcinomatosis in a Young Adult with Carcinoma Stomach
Prasanta Kumar Bhattacharya1, Md. Jamil2, Yookarin Khonglah3, Aakash Roy4, Murti V. Subrahmanya5
1 Professor and Head, Department of General Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
2 Assistant Professor, Department of General Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
3 Associate Professor, Department of Pathology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
4 Postgraduate Student, Department of General Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
5 Postgraduate Student, Department of General Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prasanta Kumar Bhattacharya, Professor and Head, Department of General Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong-793018, Meghalaya, India.
E-mail: pkbdr78@gmail.com
Pulmonary Lymphangitic Carcinomatosis (PLC) occurs in about 6-8% of patients with lung metastasis and may rarely develop in the course of gastric cancer representing a complication due to diffuse metastasis in the lymphatics of the lungs. A 29-year-old female, admitted with difficulty in breathing and productive cough for one week, was initially evaluated for respiratory tract infection. During evaluation of associated anaemia an upper gastrointestinal endoscopy showed large ulcerative growth in the lesser curvature of the stomach suggestive of carcinoma. A High Resolution Computed Tomographic (HRCT) scan of the lungs was done for evaluation of the pulmonary opacities on chest x-ray which showed nodular thickening of interlobular septa with peribronchial cuffing and fissural thickening. The biopsy of the gastric ulcer was suggestive of poorly differentiated malignancy. With the cumulative results of the investigations a diagnosis of poorly differentiated carcinoma of stomach with pulmonary metastasis as lymphangitic carcinomatosis was made. PLC is an extremely rare manifestation of metastatic gastric cancer. Though associated with an extremely poor prognosis, advanced gastric cancer in younger patients presenting with symptoms and signs of respiratory disease should alert the physician of a possible diagnosis of PLC.
Gastric cancer, Lung manifestation, Young female
Case Report
A 29-year-old female was admitted with the history of progressive weight loss over the previous three months, difficulty in breathing and minimally productive cough of one week duration. There was no history of fever, haemoptysis, palpitations or chest pain. She gave a history of contact with tuberculosis in her household. She had no history of rash, substance abuse, known allergy or chronic drug intake.
On examination she had severe pallor, with hypotension (blood pressure: 96/60 mmHg), tachycardia (pulse rate 138/min) and tachypnoea (respiratory rate: 28/min) with an oxygen saturation of 86% at room air which improved to 92% with supplemental oxygen at 4 L/min. An initial arterial blood gas analysis showed a pO2 of 67.3 mmHg, pCO2 of 28.4 mmHg and a pH of 7.52. There was no cyanosis, pedal oedema, clubbing, lymphadenopathy or ascitis. Respiratory examination revealed bilateral coarse crepitations. Except for tachycardia, cardiac examination was normal. Rest of the systemic examination was normal.
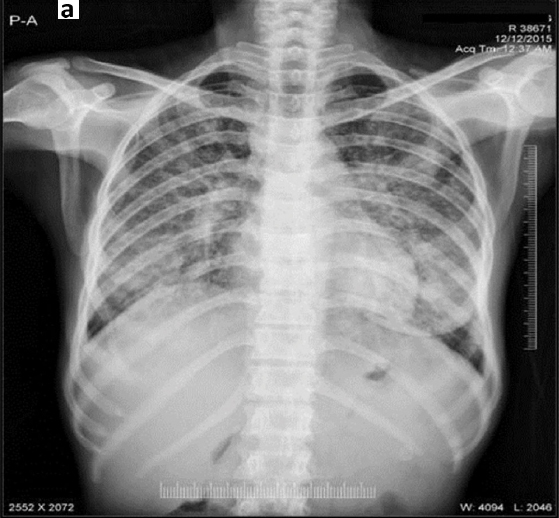
Chest x-ray showed bilateral nodular opacities in the mid and lower lung zones [Table/Fig-1]. Electrocardiography showed sinus tachycardia with right axis deviation. Initial laboratory investigations revealed anaemia with haemoglobin of 6.7gm% and a microcytic hypochromic picture, erythrocyte sedimentation rate of 37 mm/hr, platelet count of 270x103/ mm3 and total leucocyte count of 8400/ mm3 with 84% neutrophils. The mean corpuscular volume was 69 fl and mean corpuscular haemoglobin was 17 pg/cell. Serum iron was 23 ug/dl and serum ferritin was 67 ng/ml. Her renal and liver function tests, serum electrolytes and coagulogram were within normal limits. Urine analysis was normal and serology for viral markers including HIV, HBV and HCV were non-reactive. Stool routine examination was within normal limits but stool for occult blood came positive.
Chest x-ray showing bilateral opacities in the mid and lower lung zones.
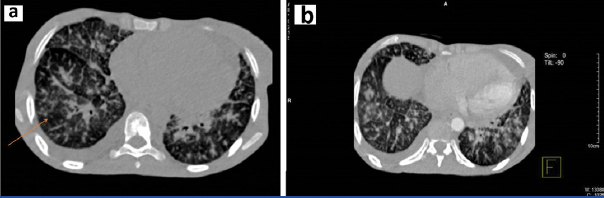
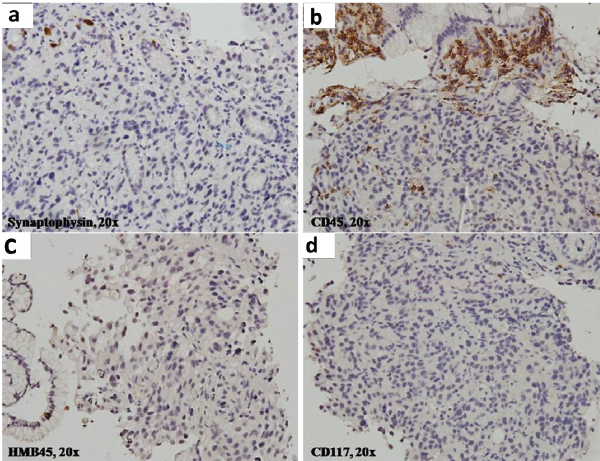
The patient was initially managed to ensure haemodynamic stability with intravenous fluid, transfusion of three units of packed red blood cells, broad spectrum antibiotics along with other symptomatic measures. Sputum analysis for acid fast bacilli was negative and bacteriological culture was sterile. However, based on the history of contact with tuberculosis in the family, anti-tubercular therapy consisting of isoniazid, rifampicin, ethambutol and pyrazinamide was started empirically. Two-dimensional echocardiogram showed dilated right atrium and right ventricle, severe Pulmonary Arterial Hypertension (PAH) with normal left ventricular ejection fraction. Stool for occult blood was positive and a consequent upper gastro-intestinal endoscopy showed a large ulcerative growth in the lesser curvature of the stomach suggestive of carcinoma of the stomach. A HRCT scan of the thorax showed nodular thickening of interlobular septa with peribronchial cuffing and fissural thickening along with minimal right sided pleural effusion [Table/Fig-2]. The biopsy from the gastric ulcer showed poorly differentiated malignancy. On immunohistochemistry, epithelial membrane antigen showed strong membranous and cytoplasmic positivity and cytokeratin showed variable positivity, confirming its epithelial origin in favour of adenocarcinoma. CD45, CD3, CD10, CD20 were negative ruling out lymphoma. CD117, HMB 45, and synaptophysin were negative ruling out gastrointestinal stromal tumour, malignant melanoma and neuroendocrine carcinoma respectively [Table/Fig-3,4 and 5].
a) HRCT scan showing nodular thickening of interlobular septa with peribronchial cuffing and fissural thickening along with minimal right sided pleural effusion; b) HRCT scan showing nodular thickening of interlobular septa with peribronchial cuffing and fissural thickening.
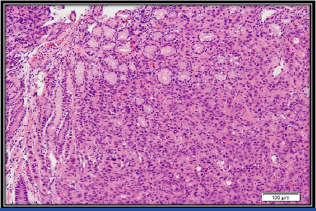
Tumour cells infiltrating the lamina propria. Intact gastric mucosal glands can be seen in the upper half (H&E Stain; 100X).
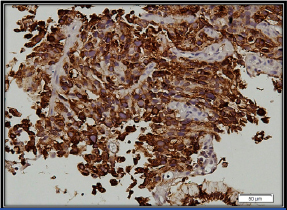
Tumour cells showing cytoplasmic positivity for cytokeratin Immunohistochemistry (IHC); 200X).
Tumour cells are negative for: a) synaptophysin; b) CD45; c) HMB45; d) CD117, ruling out neuroendocrine tumour, lymphoma, melanoma and gastrointestinal stromal tumours, respectively (IHC; 200X).
Based on the clinical presentation and investigations, a diagnosis of poorly differentiated carcinoma of stomach with pulmonary metastasis in the form of lymphangitic carcinomatosis was made. The patient was explained about the nature and prognosis of the disease and the need for palliative chemotherapy. However, she refused further investigations and therapy and had to be discharged on request.
Discussion
The term lymphangitic carcinomatosis was first coined by Troisier in 1873 to describe a condition of diffuse infiltration of the lymphatics of both the lungs by malignant cells [1]. The condition has a greater predilection for males (male: female= 60:40) and in contrast to other malignancies has a greater propensity to affect a comparatively younger population [1]. Although, there are chances that any metastatic neoplasm may cause PLC, the commonest locations of the primary tumour are lungs, breasts, pancreas, stomach and prostate [1]. PLC may develop as metastasis of gastric cancer, and may be the sole manifestation of an asymptomatic gastric malignancy [2,3]. In a previous retrospective study in 43 patients with PLC, 23 patients had primary lung cancer, nine breast cancers, eight with large intestinal carcinoma and six gastric carcinoma [4]. The pathogenesis of PLC is generally explained by two distinctive theories, the first of which states that there is haematogenous metastasis producing a form of obliterative endarteritis and subsequent extravasation of the tumour cells through vessel walls into the perivascular lymphatics. The second theory proposes that a diffuse retrograde permeation and embolization of lymphatics occurs after preliminary involvement of the pulmonary hilar lymph nodes [5]. The presentation may occur either after the diagnosis of the primary cancer has been made as observed in previous reports [2]. or be the first presenting symptom overall as in our case. Similar to our case previous reports have documented patients primarily with complaints breathing difficulty without any prominent local symptoms of gastric malignancy [5].
On chest radiography abnormalities are found to be more frequent in the right lung than in the left and in up to 30% of the cases there may be no abnormality. Our case showed bilateral infiltrates which has also been reported in previous cases [5]. No single radiological manifestation is however diagnostic and the radiological differentials include a wide variety of conditions including sarcoidosis, viral pneumonias, pulmonary oedema, radiation pneumonitis and lymphocytic interstitial pneumonia. Nodular shadows are the commonest, but diffuse shadows, hilar lymphadenopathy and pleural effusion can also be seen [2]. Computed tomographic scans are often more useful with distinctive changes like thickening and irregularity of the linear patterns and changes in a bronchoalveolar and peri-lobular pattern [1,5]. In addition to patterns in CT scans a transbronchial biopsy further assists in the diagnosis of PLC, which however could not be done in our case and is a limitation in our study.
Gastric cancer is one of the commonest cancers worldwide, accounting for about 8% of newly detected cancers [6]. Furthermore, for reasons that are unclear; there is an increasing trend in incidence in the young population in recent years [7]. Patients with primary gastric cancer and lymphangitic carcinomatosis have a usual presentation of progressive dyspnoea lasting for two to four months before definite diagnosis [8]. However, our patient had a comparatively shorter duration of presentation and progression of disease.
The treatment options for PLC are limited. The role of steroids for symptomatic relief of dyspnoea has been suggested in previous reports but is too of limited value [5]. The prognosis of patients with PLC is extremely poor with an average survival of only ninety days [1]. In a previous study comprising of six patients with a mean age of 26 years and a primary gastric tumour had a mean time of survival of 22 days after their first admission to hospital [9]. Currently there are no proven effective treatment strategies for PLC. However steroid administration may produce symptomatic improvement mainly by relieving episodes of breathlessness [1].
Conclusion
Although, PLC is a rare metastatic complication of gastric malignancies, patients with PLC should be thoroughly investigated for primary gastric malignancy. This case study highlights the fact that gastric malignancy in younger patients presenting with symptoms and signs of respiratory disease should alert the physician of a possible diagnosis of PLC and appropriate investigations should be done for an early and appropriate diagnosis.
[1]. Bruce DM, Heys SD, Eremin O, Lymphangitis carcinomatosa: a literature reviewJournal of the Royal College of Surgeons of Edinburgh 1996 41(1):7-13. [Google Scholar]
[2]. Witczak A, Prystupa A, Zamecka MO, Bilan A, Krupski W, Mosiewicz J, Pulmonary lymphangitic carcinomatosis in the course of gastric cancer-Case reportJournal of Pre-Clinical and Clinical Research 2014 8(2):116-19. [Google Scholar]
[3]. Zhuang L, Liu X, Hu C, Zhang L, Jiang G, Wu J, Pulmonary lymphangitic carcinomatosis in liver carcinoma: a rare case report and literature reviewWorld Journal of Surgical Oncology 2014 12(1):1-5. [Google Scholar]
[4]. Zhang K, Huang Y, Clinical features and diagnosis of pulmonary lymphangitic carcinomatosisAi Zheng 2006 25:1127-30. [Google Scholar]
[5]. Raja A, Seshadri RA, Sundersingh S, Lymphangitis Carcinomatosa: Report Of A Case And Review Of LiteratureIndian Journal of Surgical Oncology 2010 1(3):274-76. [Google Scholar]
[6]. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, Global cancer statisticsCA Cancer J Clin 2011 61:69-90. [Google Scholar]
[7]. Correa P, Gastric cancer: two epidemics?Dig Dis Sci 2011 56:1585-86. [Google Scholar]
[8]. Moubax K, Wuyts W, Vandecaveye V, Prenen H, Pulmonary lymphangitic carcinomatosis as a primary manifestation of gastric carcinoma in a young adult: a case report and review of the literatureBMC Research Notes 2012 5(1):638-42. [Google Scholar]
[9]. Kwee TC, Takahara T, Ochiai R, Katahira K, Van Cauteren M, Imai Y, Whole-body diffusion-weighted magnetic resonance imagingEur J Radiol 2009 70:409-17. [Google Scholar]