Platelets not only function as a factor in haemostasis, but also helps in regeneration of tissues. They contain various growth factors, such as Platelet Derived Growth Factor (PDGF) and transforming growth factor beta (TGF-β) [1,2]. Platelet Rich Plasma (PRP), the concentrate of platelets from peripheral blood, is appreciated as safe and cost-effective source of growth factors [3,4]. To process PRP, peripheral blood is collected in a syringe containing anti-coagulant and then it is centrifuged twice. PRP can be directly applied as watery liquid or be used after gelation.
Bone marrow cells have been estimated as an effective material for wound healing and regenerative medicine [5-7]. Number of platelets in bone marrow aspirate is the same as those in peripheral blood. And the growth factor levels are the same. Nishimoto S et al., and Nishimoto S et al., demonstrated the procedure to process PRP from peripheral blood for bone marrow aspirate, and concluded that bone marrow cells and platelets can be concentrated simultaneously [8,9]. It was called bone marrow derived platelet rich plasma (bm-PRP) and is been used clinically [10,11].
Platelet rich fibrin (PRF) was developed by Choukroun et al., as a new generation of platelet concentration from peripheral blood [12]. The procedure to make PRF is very simple [13]. Peripheral blood, taken without anticoagulant, is poured into a glass centrifugation tube and centrifuged. The glass surface, negatively charged, will activate Hageman factor (factor XII), which is the starting point of intrinsic coagulation pathway. Polymerization of fibrin occurs and yellow-red layered gel will be obtained. Top yellow layer is cut out as PRF. Positive clinical reports about PRF are increasing. Moderate firmness of the gel makes it easy to handle. Nishimoto S et al., measured growth factors; PDGF and TGF-β, in PRF. It revealed that growth factors are surely concentrated in PRF, but not equally distributed throughout PRF [14]. In the same report, it was shown histologically that nucleated cells and platelets are packed along the yellow-red interface. It implies that, very high concentration of platelets, which contain growth factors and nucleated cells, potentially work for tissue regeneration, can be obtained by taking out yellow-red interface.
Objective of study is to make PRF from bone marrow aspirate and study the histological aspects.
Materials and Methods
Bone Marrow Aspiration
Japanese white rabbits (around 2 kg) were housed under standard conditions and treated according to the protocol (No. 12-066) approved by animal care and use committee of Hyogo College of Medicine.
The rabbits were anaesthetized with inhalation of 3% isoflurane. Iliac crest of the animal was stabbed with Komiya’s bone marrow needle (Kurita, Tokyo, Japan) [9]. Two ml of bone marrow aspirate was obtained in a 2.5 ml polypropylene syringe containing 0.2 ml of anticoagulant (ACD-A solution: 2.2 % sodium citrate, 0.8 % citrate, 2.2 % glucose) (Terumo, Tokyo, Japan), or in a syringe without anticoagulant.
Processing bm-PRF
A: Normal bm-PRF: Bone marrow aspirate without anticoagulant was poured into a glass tube (IWAKI 9831-1207, Asahi Glass, Tokyo, Japan). The tube was immediately spun in a centrifuging machine (6200 Kubota, Tokyo, Japan) with 400 g for 10 minutes at room temperature.
B: Normal bm-PRF after filtration: Bone marrow aspirate without anticoagulant was filtered with 30-micron cell filter (Filcon S, As One, Osaka, Japan) and poured into a glass tube. The tube was immediately centrifuged with 400 g for 10 minutes at room temperature.
C: bm-PRF made from bone marrow aspirate with anticoagulant and calcium chloride: Bone marrow aspirate with anticoagulant was poured into a glass tube and 5 μl of 10% calcium chloride was added. Then the tube was centrifuged with 800g for 10 minutes at 40C.
D: bm-PRF made from filtered bone marrow aspirate with anticoagulant and calcium chloride: Bone marrow aspirate with anticoagulant was filtered with 30-micron cell filter and poured into a glass tube. 5 μl of 10% calcium chloride was added and centrifuged with 800g for 10 minutes at 40C.
Gross Observation
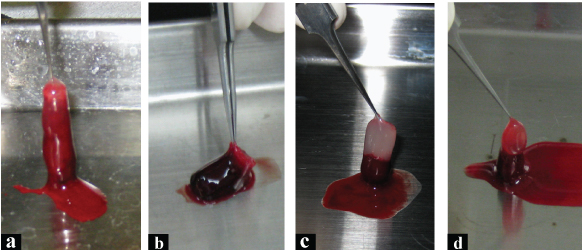
Interior wall of the tube was scraped with a spatula. Gel in the tube was taken out and observed [Table/Fig-1].
Macroscopic view of the gels, taken out from the glass tubes. a) Normal bm-PRF. Border of white and red zones is ambiguous. b) Normal bm-PRF after filtration. Volume of bm-PRF was considerably small. Top part color is more sanguine than A. c) bm-PRF made from bone marrow aspirate with anticoagulant and calcium chloride. Border of white and red zone was distinct. d) bm-PRF made from filtered bone marrow aspirate with anticoagulant and calcium chloride. Top part color was reddish.
Histological Observation
Gels (A,C,D) were fixed with buffered 10% formaldehyde. Paraffin embedded sections were made and stained with Giemsa stain solution (Wako Pure Chemical industries, Osaka, Japan). Histological images were taken through a microscope (PROVIOS AX80, Olympus, Tokyo, Japan) and a digital camera (DP72, Olympus, Tokyo, Japan). Panoramic pictures were composed with Image Composite Editor (Microsoft, Redmond, WA, USA).
Results
Gross Observation
A: Normal bm-PRF: Two-toned (white and red) gel was obtained. Border of white and red zone was indistinct.
B: Normal bm-PRF after filtration: Red gel with slightly orange top was obtained Border of the colours was ambiguous. Volume was considerably smaller than normal bm-PRF. This sample was not proceeded to histological analysis as this method cannot be the candidate for the aim.
C: bm-PRF made from bone marrow aspirate with anticoagulant and calcium chloride: Two-toned (white and red) gel was obtained. Border of white and red zone was sharply demarcated.
D: bm-PRF made from filtered bone marrow aspirate with anticoagulant and calcium chloride: Two toned (orange and white) gel was obtained. Border of two colours was sharp. Volume was smaller than bm-PRF made from bone marrow aspirate with anticoagulant and CaCl2.
Histological Observation
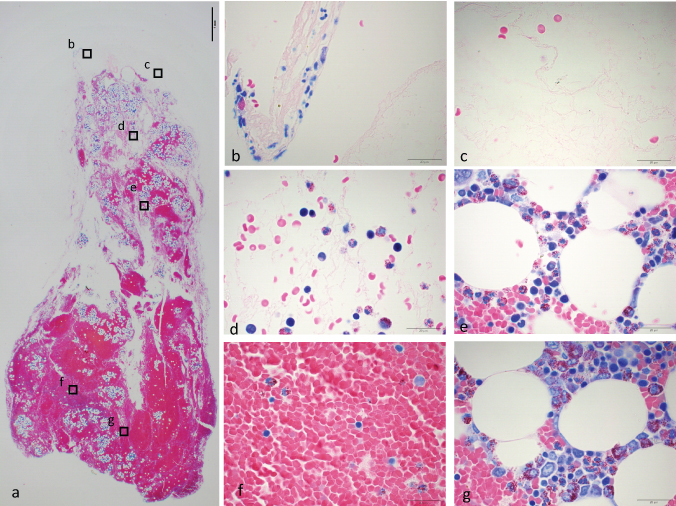
A:Normal bm-PRF [Table/Fig-2]: On top surface of the gel, small number of nucleated cells was seen. In white area, amorphous stroma dominated and scattered red blood cells were seen. Density of red blood cell was low in upper part of the gel, and high in lower part. Nucleated cells were observed throughout the red area. Cells with adipose vacuole were also seen throughout the red area. Aggregation of platelets scattered here and there.
Formaldehyde fixed bm-PRF made from bone marrow aspirate without anti-coagulant (A). a) Panoramic image taken with x2 objective lens. A bar indicates 1 mm. Border between clear and red area is unclear. Islets with vacuoles scattered here and there. Alphabets indicate where high magnification images were taken. b–g: Images taken with x100 objective lens with oil immersion. b) Top surface of the gel. Some nucleated cells are observed. c) Clear part of the gel. Amorphous stroma with low number of cells were observed. d) Scattered red blood cells and nucleated cells were seen. e) Islet with vacuoles seen in middle part of the gel. Nucleated cells and platelets aggregate around adipose cells. f) Red clot area. Red blood cells with low number of nucleated cells were packed. g) Islet with vacuole seen in lower part of the gel.
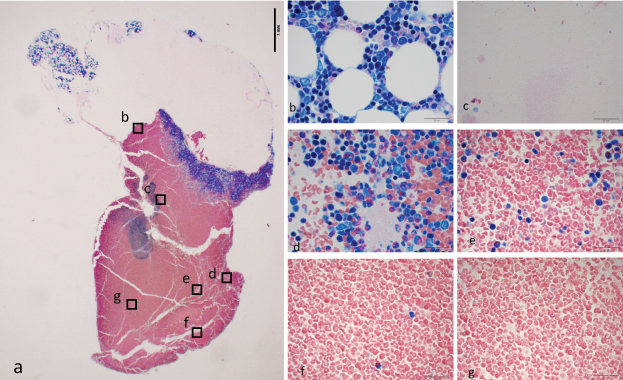
C: bm-PRF made from bone marrow aspirate with anticoagulant and calcium chloride [Table/Fig-3]: On top surface of the gel, Adipose cells and some number of nucleated cells were observed. There was a clear amorphous zone with low density of cells, next to the surface. Most of the red blood cells were situated lower part of the gel. Along the border between white and red area, highly-packed nucleated cells and platelets were observed.
Bone marrow aspirate with anti-coagulant, centrifuged immediately after calcium chloride was added (C). a) Formaldehyde fixed gel taken with x2 objective lens. A bar indicates 1 mm. Around surface of the gel, aggregation with vacuoles were observed. There was a blue band between clear and red area. b–g: Images taken with x100 objective lens with oil immersion. Bars indicate 20 μm b) Nucleated cells aggregated around adipose cells, on the top of the gel. c) Amorphous zone with low number of cells. d) The blue band was composed of packed nucleated cells and platelets. e) Number of nucleated cells were lower in just below the blue band. f) Middle part of the gel. g) Lower part of the gel.
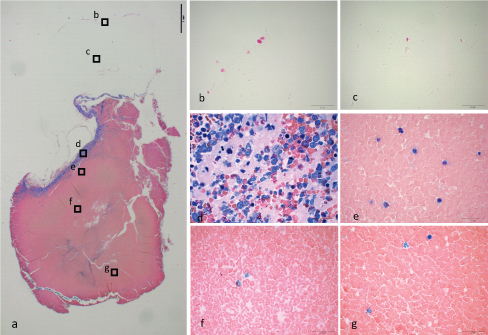
D: bm-PRF made from filtered bone marrow aspirate with anti-coagulant and calcium chloride [Table/Fig-4]: On top surface, very low density of cells could be seen. Upper part of the gel was occupied with amorphous stroma with low density of cells. Lower part was occupied with red blood cells. The border consisted of dense nucleated cells and platelets. The band of nucleated cells and platelets was narrower than C.
Bone marrow aspirate with anti-coagulant was filtered with 30 μm cell filter (D). It was centrifuged immediately after calcium chloride was added. a) Formaldehyde fixed gel taken with x2 objective lens. A bar indicates 1 mm. The area with and without color was clearly divided. The blue band just below the interface was narrower than C. b) Top surface of the gel. Number of cells was low. c) Very low number of cells could be seen in clear fibrin network. d) Highly packed platelets and nucleated cells consisted the interface band. e) Some nucleated cells were observed just below the band. f) Middle part of the gel. g) Lower part of the gel.
Discussion
PRF made from peripheral blood has been used clinically and positive results have been reported [15,16]. It is easy to make and easy to handle with its moderate firmness. In our previous report, we histologically confirmed that platelets, which contain growth factors and nucleated cells, are highly packed along the yellow-red border [14]. We also have experimental [9] and clinical [10,11] experience of simultaneous concentration of platelets and nucleated cells from bone marrow aspirate. The aim of this study was to investigate whether the easiness of making and handling of PRF can be applied by working with bone marrow aspirate.
Making PRF from peripheral blood is very easy and straight forward. It is nothing more than to centrifuge blood in a glass tube [13]. Firstly, making of bm-PRF was attempted with the same simple procedure (A). Though the border between two-toned colour was ambiguous. Histologically, Platelets and nucleated cells were not as packed as was seen in PRF from peripheral blood [14]. In some area, nucleated cells were seen with adipose cells. To eliminate those adipose cells, which have larger diameter than nucleated cells, filtering with 30-micron cell filter before centrifugation was attempted (B). The filters were easily clogged. The yield of product was significantly lower. This sample was not proceeded to histological analysis. For next experiment, bone marrow was aspirated with anti-coagulant. Calcium chloride was added immediately before the centrifugation (C). Macroscopically, clearly two-tone defined gel was observed. Histologically, Platelets and nucleated cells were packed along the border. On top surface of the gel, there was the gathering of nucleated cells with adipose cells. Lastly, bone marrow aspirate with anti-coagulant was filtered before centrifugation and gelation (D). Haemolyzation was seen. Volume of gel obtained was lower than C. Microscopically, no adipose cell was observed. The nucleated cells were packed with platelets as a band along the border. The band was narrower than C.
Simple application of processing PRF from peripheral blood to bone marrow aspirate did not work well. The bone marrow aspirate coagulated before the cells in it were distributed with respective specific gravity. With our current technique of bone marrow aspiration from rabbit iliac crest, the aspirate has faster coagulation tendency than peripheral blood from human vein. Bone marrow stroma exist in chambers finely segmented by bony trabecula. Stabbing bone with a needle break those chamber wall, which can be a trigger for coagulation cascade. Aspirating bone marrow aspirate with anti-coagulant and adding calcium chloride immediately before the centrifugation could work out the problem.
To obtain the same kind of product, there is another procedure to make PRP from bone marrow aspirate and let it coagulate afterward. There are some different ways to process PRP. One of the most common ways is double-spin method. It usually takes 30 minutes. Making bm-PRF does not take that long and at the end of centrifugation, gelation has already done. By cutting out yellow-red border, gel with highly-condensed platelet and nucleated cells can be obtained.
Bone marrow aspirate contain some number of adipose cells. They float in water with their light specific gravity. They have sticky surface that capture nucleated cells and let them float on top of the gel. Top part of the gel may be used as another part that contains high concentration of nucleated cells. Attempt to eliminate those adipose cells succeed with cost of losing yield amount and haemolysis. For clinical use, paying the cost does not seem worth.
Conclusion
PRF from bone marrow aspirate can be made, but its not the same simple procedure that is used to make PRF from peripheral blood. PRF can made from bone marrow aspirate by adding anti-coagulant in aspiration and reversed with calcium chloride just before centrifugation.